当前位置:
X-MOL 学术
›
Results Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure–activity relationship studies of 5-(Pyridin-3-yl)-1H-indole-4,7-diones as indoleamine 2,3-dioxygenase 1 inhibitors
Results in Chemistry ( IF 2.5 ) Pub Date : 2023-12-24 , DOI: 10.1016/j.rechem.2023.101285 Jiawei Zhang , Jingyi Liu , Kaimin Kong , Xingzhou Li , Qian Zhang
Results in Chemistry ( IF 2.5 ) Pub Date : 2023-12-24 , DOI: 10.1016/j.rechem.2023.101285 Jiawei Zhang , Jingyi Liu , Kaimin Kong , Xingzhou Li , Qian Zhang
|
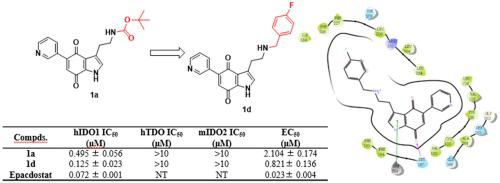
Indoleamine 2,3-dioxygenase 1 (IDO1) is the key enzyme that catalyzes the conversion of -tryptophan (Trp) to -formyl-kynurenine in the tryptophan-kynurenine (Trp-Kyn) pathway. Overexpression of IDO1 contributes to the depletion of Trp and the accumulation of Kyn, which can result in tumor immune escape. Inhibition of IDO1 can restore the host immune response to eradicate cancer cells. 5-(Pyridin-3-yl)-1-indole-4,7-dione was developed as the scaffold for a type of IDO1 inhibitors from simplification of the structure of exiguamine A in our previous work. In the present study, we designed and synthesized a series of compounds with different side-chain substituents, and linkers of varying lengths, at the 3-position, to design compounds with an aryl motif that can occupy pocket B of the IDO1 protein. Most compounds exhibited potent IDO1 inhibitory activity with IC values at the micromolar level, and 3-(2-((4-fluorobenzyl)amino)ethyl)-5-(3-pyridyl)-1-indole-4,7-dione () displayed the most potent inhibition with a half-maximal inhibitory concentration (IC) value of 0.125 μM in an enzymatic assay and a half-maximal effective concentration (EC) value of 0.821 μM in a cellular assay. Compound showed higher selectivity for IDO1 over indoleamine 2,3-dioxygenase 2 and tryptophan 2,3-dioxygenase at the effective concentration. Molecular docking studies and molecular dynamic simulations suggested that the phenyl ring of these inhibitors can enter into pocket B of IDO1 and interact with the residue Phe 226 through hydrophobic interactions.
中文翻译:

5-(Pyridin-3-yl)-1H-indole-4,7-diones 作为吲哚胺 2,3-双加氧酶 1 抑制剂的构效关系研究
吲哚胺 2,3-双加氧酶 1 (IDO1) 是色氨酸-犬尿氨酸 (Trp-Kyn) 途径中催化 -色氨酸 (Trp) 转化为 -甲酰基-犬尿氨酸的关键酶。 IDO1 的过度表达会导致 Trp 的消耗和 Kyn 的积累,从而导致肿瘤免疫逃逸。抑制IDO1可以恢复宿主免疫反应,从而消灭癌细胞。在我们之前的工作中,通过简化 exiguamine A 的结构,开发了 5-(Pyridin-3-yl)-1-indole-4,7-dione 作为一种 IDO1 抑制剂的支架。在本研究中,我们设计并合成了一系列在3位具有不同侧链取代基和不同长度连接子的化合物,以设计具有可占据IDO1蛋白口袋B的芳基基序的化合物。大多数化合物表现出有效的 IDO1 抑制活性,IC 值在微摩尔水平,3-(2-((4-氟苄基)氨基)乙基)-5-(3-吡啶基)-1-吲哚-4,7-二酮 ( )显示出最有效的抑制作用,酶测定中的半最大抑制浓度 (IC) 值为 0.125 μM,细胞测定中的半最大有效浓度 (EC) 值为 0.821 μM。在有效浓度下,化合物对 IDO1 的选择性高于吲哚胺 2,3-双加氧酶 2 和色氨酸 2,3-双加氧酶。分子对接研究和分子动力学模拟表明这些抑制剂的苯环可以进入IDO1的口袋B并通过疏水相互作用与残基Phe 226相互作用。
更新日期:2023-12-24
中文翻译:

5-(Pyridin-3-yl)-1H-indole-4,7-diones 作为吲哚胺 2,3-双加氧酶 1 抑制剂的构效关系研究
吲哚胺 2,3-双加氧酶 1 (IDO1) 是色氨酸-犬尿氨酸 (Trp-Kyn) 途径中催化 -色氨酸 (Trp) 转化为 -甲酰基-犬尿氨酸的关键酶。 IDO1 的过度表达会导致 Trp 的消耗和 Kyn 的积累,从而导致肿瘤免疫逃逸。抑制IDO1可以恢复宿主免疫反应,从而消灭癌细胞。在我们之前的工作中,通过简化 exiguamine A 的结构,开发了 5-(Pyridin-3-yl)-1-indole-4,7-dione 作为一种 IDO1 抑制剂的支架。在本研究中,我们设计并合成了一系列在3位具有不同侧链取代基和不同长度连接子的化合物,以设计具有可占据IDO1蛋白口袋B的芳基基序的化合物。大多数化合物表现出有效的 IDO1 抑制活性,IC 值在微摩尔水平,3-(2-((4-氟苄基)氨基)乙基)-5-(3-吡啶基)-1-吲哚-4,7-二酮 ( )显示出最有效的抑制作用,酶测定中的半最大抑制浓度 (IC) 值为 0.125 μM,细胞测定中的半最大有效浓度 (EC) 值为 0.821 μM。在有效浓度下,化合物对 IDO1 的选择性高于吲哚胺 2,3-双加氧酶 2 和色氨酸 2,3-双加氧酶。分子对接研究和分子动力学模拟表明这些抑制剂的苯环可以进入IDO1的口袋B并通过疏水相互作用与残基Phe 226相互作用。







































 京公网安备 11010802027423号
京公网安备 11010802027423号