当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Solution Stoichiometry on BaSO4 Crystallization from Turbidity Measurements and Modeling
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-12-22 , DOI: 10.1021/acs.iecr.3c03612 V F D Peters 1 , A Baken 2, 3 , S Y M H Seepma 1 , J A Koskamp 1 , A Fernández-Martínez 2 , A E S van Driessche 2, 4 , M Wolthers 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-12-22 , DOI: 10.1021/acs.iecr.3c03612 V F D Peters 1 , A Baken 2, 3 , S Y M H Seepma 1 , J A Koskamp 1 , A Fernández-Martínez 2 , A E S van Driessche 2, 4 , M Wolthers 1
Affiliation
|
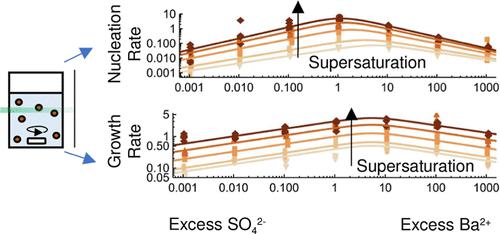
The impact of solution stoichiometry on the nucleation and growth of BaSO4 was studied by measuring solution transmittance and subsequent fitting to a crystallization model. Our results show that a large excess of either Ba2+ or SO42– ions inhibits both the nucleation and growth of BaSO4. However, for a small excess of Ba2+, the growth is enhanced. The dependence of nucleation and growth rates on supersaturation and solution stoichiometry was captured by a semiempirical rate model. Hence, the solution stoichiometry is a highly relevant parameter while studying all aspects of BaSO4 crystallization, and it could be worthwhile to examine other minerals similarly.
中文翻译:

浊度测量和建模中溶液化学计量对 BaSO4 结晶的影响
通过测量溶液透射率并随后拟合结晶模型,研究了溶液化学计量对 BaSO 4成核和生长的影响。我们的结果表明,大量过量的Ba 2+或SO 4 2–离子会抑制BaSO 4的成核和生长。然而,对于稍微过量的Ba 2+ ,生长得到增强。半经验速率模型捕获了成核和生长速率对过饱和度和溶液化学计量的依赖性。因此,在研究 BaSO 4结晶的各个方面时,溶液化学计量是一个高度相关的参数,并且值得对其他矿物进行类似的检查。
更新日期:2023-12-22
中文翻译:

浊度测量和建模中溶液化学计量对 BaSO4 结晶的影响
通过测量溶液透射率并随后拟合结晶模型,研究了溶液化学计量对 BaSO 4成核和生长的影响。我们的结果表明,大量过量的Ba 2+或SO 4 2–离子会抑制BaSO 4的成核和生长。然而,对于稍微过量的Ba 2+ ,生长得到增强。半经验速率模型捕获了成核和生长速率对过饱和度和溶液化学计量的依赖性。因此,在研究 BaSO 4结晶的各个方面时,溶液化学计量是一个高度相关的参数,并且值得对其他矿物进行类似的检查。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号