当前位置:
X-MOL 学术
›
ACS Mater. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NIR-II-/Tumor Acidity-Triggered Nanoplatform for Precise Chemodynamic/Photodynamic/Hypoxia-Activated Chemo Trimodal Synergistic Therapy
ACS Materials Letters ( IF 9.6 ) Pub Date : 2023-12-22 , DOI: 10.1021/acsmaterialslett.3c01070 Biao-Qi Chen 1 , Yi Zhao 1 , Yu-Jing Pan 1 , Da-Gui Zhang 1 , Hao Liu 1 , Yu-Hong Shi 1 , Chang-Yong Li 1 , Ranjith Kumar Kankala 1 , Yang Zhang 2, 3 , Shi-Bin Wang 1 , Gang Liu 3 , Ai-Zheng Chen 1
ACS Materials Letters ( IF 9.6 ) Pub Date : 2023-12-22 , DOI: 10.1021/acsmaterialslett.3c01070 Biao-Qi Chen 1 , Yi Zhao 1 , Yu-Jing Pan 1 , Da-Gui Zhang 1 , Hao Liu 1 , Yu-Hong Shi 1 , Chang-Yong Li 1 , Ranjith Kumar Kankala 1 , Yang Zhang 2, 3 , Shi-Bin Wang 1 , Gang Liu 3 , Ai-Zheng Chen 1
Affiliation
|
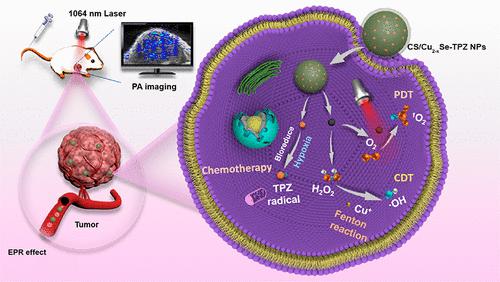
Engineering stimuli-responsive nanoplatforms for multimodal therapeutics to combat the diversity, complexity, and heterogeneity of malignant tumors has emerged as a new research focus. Herein, chitosan (CS)-based nanoplatforms coloaded with copper selenide quantum dots (Cu2–xSe QDs) and tirapazamine (TPZ) were prepared by a facile ion-cross-linking method (CS/Cu2–xSe-TPZ NPs) to achieve stimuli-responsive precise chemodynamic-/photodynamic-/chemotherapy-based trimodal tumor therapy. Triggered by the endogenous tumor acidic microenvironment and the exogenous second near-infrared (NIR-II) light, Cu2–xSe QDs efficiently generated dreadful radicals (•OH and 1O2) to achieve efficient Cu2–xSe-based synergistic chemodynamic therapy (CDT) and photodynamic therapy (PDT). Subsequently, PDT-induced hypoxia activated the cytotoxic potential of TPZ, thus inciting a hypoxia-specific chemotherapeutic effect. The synergistic tumor suppression effect of the trimodal therapy could overcome the hindrance of the tumor microenvironment, showing remarkable therapeutic effects in vitro and in vivo. Together, this work unveiled the potential of engineered stimuli-responsive nanoplatforms by integrating NIR-II-induced PDT, acidity-boosted CDT, and hypoxia-activated chemotherapy, offering potential solutions to current challenges in cancer therapy.
中文翻译:

NIR-II-/肿瘤酸度触发的纳米平台,用于精确的化学动力学/光动力学/缺氧激活化疗三模式协同治疗
设计用于多模式治疗的刺激响应纳米平台来对抗恶性肿瘤的多样性、复杂性和异质性已成为新的研究热点。在此,通过简单的离子交联方法(CS/Cu 2– x Se-TPZ NPs )制备了共负载硒化铜量子点(Cu 2– x Se QDs)和替拉扎明(TPZ)的壳聚糖(CS)纳米平台。 )以实现基于刺激响应的精确化学动力学/光动力学/化疗的三模式肿瘤治疗。在内源性肿瘤酸性微环境和外源性第二近红外(NIR-II)光的触发下,Cu 2– x Se QDs有效地产生可怕的自由基(·OH和1 O 2),以实现高效的Cu 2– x Se基协同作用化学动力疗法(CDT)和光动力疗法(PDT)。随后,PDT 诱导的缺氧激活了 TPZ 的细胞毒性潜力,从而引发缺氧特异性化疗效果。三联疗法的协同抑瘤作用可以克服肿瘤微环境的阻碍,在体外和体内均表现出显着的治疗效果。总之,这项工作通过整合 NIR-II 诱导的 PDT、酸度增强 CDT 和缺氧激活化疗,揭示了工程刺激响应纳米平台的潜力,为当前癌症治疗的挑战提供了潜在的解决方案。
更新日期:2023-12-22
中文翻译:

NIR-II-/肿瘤酸度触发的纳米平台,用于精确的化学动力学/光动力学/缺氧激活化疗三模式协同治疗
设计用于多模式治疗的刺激响应纳米平台来对抗恶性肿瘤的多样性、复杂性和异质性已成为新的研究热点。在此,通过简单的离子交联方法(CS/Cu 2– x Se-TPZ NPs )制备了共负载硒化铜量子点(Cu 2– x Se QDs)和替拉扎明(TPZ)的壳聚糖(CS)纳米平台。 )以实现基于刺激响应的精确化学动力学/光动力学/化疗的三模式肿瘤治疗。在内源性肿瘤酸性微环境和外源性第二近红外(NIR-II)光的触发下,Cu 2– x Se QDs有效地产生可怕的自由基(·OH和1 O 2),以实现高效的Cu 2– x Se基协同作用化学动力疗法(CDT)和光动力疗法(PDT)。随后,PDT 诱导的缺氧激活了 TPZ 的细胞毒性潜力,从而引发缺氧特异性化疗效果。三联疗法的协同抑瘤作用可以克服肿瘤微环境的阻碍,在体外和体内均表现出显着的治疗效果。总之,这项工作通过整合 NIR-II 诱导的 PDT、酸度增强 CDT 和缺氧激活化疗,揭示了工程刺激响应纳米平台的潜力,为当前癌症治疗的挑战提供了潜在的解决方案。






























 京公网安备 11010802027423号
京公网安备 11010802027423号