Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2023-12-22 , DOI: 10.1016/j.aca.2023.342156 Yuanyuan Li 1 , Shaorong Li 1 , Yongli Wu 1 , Yulong Ma 1 , Wenxin Ji 1 , Yonggang Sun 1 , Keren Shi 1
|
Background
Molecular shape selectivity, based on the size and shape parameters of the molecule, such as length and planarity, is a separation process that can be used for compounds with restricted shapes, such as isomers. The separation of geometric isomers is challenging because these compounds have similar physicochemical properties but differ slightly in molecular shape. The ability to separate and quantify these isomers is important in high performance liquid chromatography (HPLC), which is one of the most widely used techniques in separation science today, because the shape of the molecule has a strong influence on biological processes.
Results
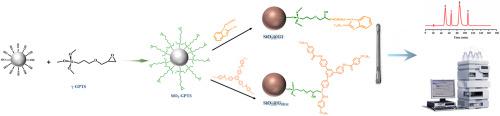
We prepared symmetrical discoidal dendrimeric organomolecule gelators (GSDM) and o-phenylenediamine-derived low-molecular-weight dendrimeric organomolecule gelators (G1) and bonded them to silica surfaces. The dendritic organic compound-grafted silica (SiO2@GSDM and SiO2@G1) was used as HPLC stationary phases for the separation of shape-restricted isomers of polycyclic aromatic hydrocarbons (PAHs), carotenoids and tocopherols. The two phases exhibit a very high molecular shape selectivity compared to the commercially available alkyl phases. There are differences in molecular shape selectivity between the two stationary phases. Changes in the chemical structure of dendritic organic compounds can alter the orientation of the molecules, as well as changes in the molecular recognition ability. It was found that SiO2@GSDM has high molecular linear selectivity for PAHs at different temperatures, even at 50 °C. The planar selectivity of SiO2@GSDM was better for triphenylene and o-terphenyl benzenes compared to SiO2@G1.
Significance
This separation behavior may be attributed to the combined effect of weak interaction centers, which allowed the effective separation of bioactive and shape-restricted isomers through multiple interactions. Furthermore, SiO2@GSDM showed better separation of tocopherols and carotenoids, suggesting that the backbone and ordered structure of organic molecular gelators is an effective way to improve the shape selectivity of the molecules, whereas the molecular orientation of the functional groups influences the separation mechanism of the shape-restricted isomers.
中文翻译:

两种树枝状有机相在二氧化硅上的固定化及其对多环芳烃、生育酚和类胡萝卜素异构体的分子形状识别
背景
分子形状选择性基于分子的大小和形状参数(例如长度和平面度),是一种可用于形状受限的化合物(例如异构体)的分离过程。几何异构体的分离具有挑战性,因为这些化合物具有相似的物理化学性质,但分子形状略有不同。分离和定量这些异构体的能力对于高效液相色谱 (HPLC) 非常重要,高效液相色谱是当今分离科学中使用最广泛的技术之一,因为分子的形状对生物过程有很大影响。
结果
我们制备了对称盘状树枝状有机分子凝胶剂(G SDM )和邻苯二胺衍生的低分子量树枝状有机分子凝胶剂(G1),并将它们粘合到二氧化硅表面。树枝状有机化合物接枝二氧化硅(SiO 2 @G SDM和 SiO 2 @G1)用作 HPLC 固定相,用于分离多环芳烃(PAH)、类胡萝卜素和生育酚的限形异构体。与市售烷基相相比,这两个相表现出非常高的分子形状选择性。两个固定相之间的分子形状选择性存在差异。树枝状有机化合物化学结构的变化可以改变分子的取向,以及分子识别能力的变化。研究发现SiO 2 @G SDM在不同温度下,甚至在50℃下对PAHs均具有较高的分子线性选择性。与SiO 2 @G1 相比,SiO 2 @G SDM对苯并菲和邻三联苯的平面选择性更好。
意义
这种分离行为可能归因于弱相互作用中心的综合作用,从而可以通过多重相互作用有效分离生物活性异构体和形状限制异构体。此外,SiO 2 @G SDM对生育酚和类胡萝卜素表现出更好的分离,这表明有机分子凝胶剂的主链和有序结构是提高分子形状选择性的有效途径,而官能团的分子取向影响分离。形状限制异构体的机制。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号