Cell ( IF 45.5 ) Pub Date : 2023-12-21 , DOI: 10.1016/j.cell.2023.11.025
Martin Wilkinson 1 , Yong Xu 1 , Dev Thacker 1 , Alexander I P Taylor 1 , Declan G Fisher 1 , Rodrigo U Gallardo 1 , Sheena E Radford 1 , Neil A Ranson 1
|
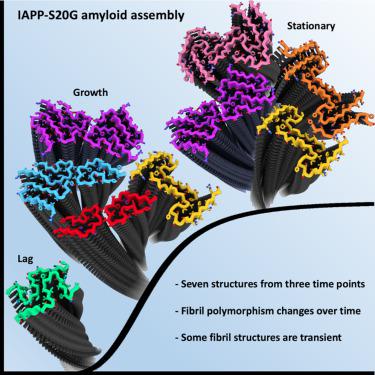
Cryoelectron microscopy (cryo-EM) has provided unprecedented insights into amyloid fibril structures, including those associated with disease. However, these structures represent the endpoints of long assembly processes, and their relationship to fibrils formed early in assembly is unknown. Consequently, whether different fibril architectures, with potentially different pathological properties, form during assembly remains unknown. Here, we used cryo-EM to determine structures of amyloid fibrils at different times during in vitro fibrillation of a disease-related variant of human islet amyloid polypeptide (IAPP-S20G). Strikingly, the fibrils formed in the lag, growth, and plateau phases have different structures, with new forms appearing and others disappearing as fibrillation proceeds. A time course with wild-type hIAPP also shows fibrils changing with time, suggesting that this is a general property of IAPP amyloid assembly. The observation of transiently populated fibril structures has implications for understanding amyloid assembly mechanisms with potential new insights into amyloid progression in disease.
中文翻译:

淀粉样蛋白组装过程中原纤维多晶型物的结构演化
冷冻电子显微镜(cryo-EM)为淀粉样蛋白原纤维结构(包括与疾病相关的结构)提供了前所未有的见解。然而,这些结构代表了长组装过程的终点,并且它们与组装早期形成的原纤维的关系尚不清楚。因此,在组装过程中是否形成具有潜在不同病理特性的不同原纤维结构仍然未知。在这里,我们使用冷冻电镜来确定与疾病相关的人胰岛淀粉样多肽变体 (IAPP-S20G)体外纤维颤动期间不同时间的淀粉样原纤维的结构。引人注目的是,在滞后期、生长期和平台期形成的原纤维具有不同的结构,随着原纤维颤动的进行,新的形式出现,而其他形式则消失。野生型 hIAPP 的时间进程也显示原纤维随时间变化,表明这是 IAPP 淀粉样蛋白组装的一般特性。对瞬时聚集的原纤维结构的观察对于理解淀粉样蛋白组装机制具有重要意义,并对疾病中淀粉样蛋白的进展具有潜在的新见解。


































 京公网安备 11010802027423号
京公网安备 11010802027423号