Polyhedron Pub Date : 2023-12-15 , DOI: 10.1016/j.poly.2023.116797 Bojan Kozlevčar , Maja Tihomirović , Marta Počkaj , Zvonko Jagličić
|
A cobalt(II/III) ionic compound with bridging tartaric acid (H4L) anion (CH6N3)3[Co2II/III(L)2(H2O)2]·4H2O 1 was synthesized. The crystallites were isolated from highly alkaline aqueous solution in the presence of guanidinium cations (CH6N3)+ being then incorporated as counter ions.
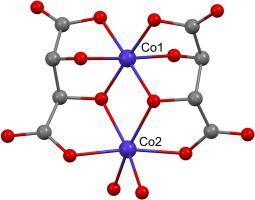
A determined structure reveals the dinuclear coordination anion with two cobalt centers, each within its distinctive coordination octahedron CoO6 sphere showing different Co−O bond lengths. The metal center with shorter coordination bonds is assigned as Co(III), while the other with Co(II), all in agreement with overall net charge equilibrium. This involves three guanidinium cations, two tartrate(4-) anions, the latter within the coordination anion, as well as with bond valence sums for both cobalt centers. Both tartrates (L) are triply bonded on the Co(III) center, thus enabling CoIIIO6 coordination bonds. On the other hand, only two coordination bonds are enabled by each tartrate towards Co(II), while the remaining two in CoIIO6 are completed by two terminal water molecules. The electronic spectrum shows clearly the d-d bands at 400 and 665 nm for Co(III), while at 520 nm for Co(II), for each CoO6, respectively. The temperature dependent magnetic susceptibility shows the paramagnetic behavior of the compound, due to the d7 Co(II) ions being in a high spin state, while the d6 low spin Co(III) ions are diamagnetic.
中文翻译:

二价 Co(II)/Co(III) 双核阴离子中的酒石酸桥配体
合成了桥连酒石酸(H 4 L)阴离子(CH 6 N 3 ) 3的钴(II/III)离子化合物[Co 2 II/III (L) 2 (H 2 O) 2 ]·4H 2 O 1。在胍鎓阳离子(CH 6 N 3 ) +存在下从高碱性水溶液中分离微晶,然后将其作为抗衡离子掺入。
确定的结构揭示了具有两个钴中心的双核配位阴离子,每个钴中心都在其独特的配位八面体 CoO 6球内,显示出不同的 Co−O 键长。具有较短配位键的金属中心被指定为Co(III),而另一个被指定为Co(II),所有这些都与总体净电荷平衡一致。这涉及三个胍盐阳离子、两个酒石酸(4-)阴离子(后者在配位阴离子内)以及两个钴中心的键价和。两种酒石酸盐 (L) 在 Co(III) 中心上三键结合,从而形成 Co III O 6配位键。另一方面,每个酒石酸盐仅对 Co(II) 形成两个配位键,而 Co II O 6中的其余两个配位键由两个末端水分子完成。对于每个 CoO 6,电子光谱分别清楚地显示了Co(III) 在 400 和 665 nm 处的d - d带,而 Co(II) 在 520 nm 处的 d-d 带。由于 d 7 Co(II) 离子处于高自旋状态,而 d 6低自旋 Co(III) 离子是抗磁性的,因此温度依赖性磁化率显示了化合物的顺磁行为。













































 京公网安备 11010802027423号
京公网安备 11010802027423号