Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2023-12-22 , DOI: 10.1016/j.comptc.2023.114446 Haoshan Sun , Xiaohui Zhang , Hongxi Liu , Jifan Li , Hua Wang
|
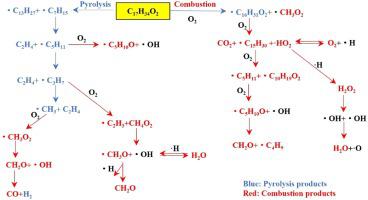
As a substitute for fossil fuel, methyl palmitate (C17H34O2) has important scientific and practical significance to study its pyrolysis and combustion mechanism. In this paper, the ReaxFF-MD method is used to research the reaction mechanism and main products of C17H34O2. The effects of temperature and O2 on the decomposition, main products and reaction paths of C17H34O2 are analyzed to determine its reaction mechanism. The results show that the reaction activation energies of C17H34O2 in pyrolysis and combustion are 201.76 kJ/mol and 139.07 kJ/mol, respectively. The effect of temperature on C17H34O2 is greater than O2 on C17H34O2 at higher temperature. The basic reaction paths for the initial decomposition of C17H34O2 is that the C C, C
C, C O, and C
O, and C H bonds are broken into smaller intermediates. In addition, the detailed distribution and generation paths of the main products C2H4, H2, H2O, CH2O, and so on are depicted to construct the reaction mechanism of C17H34O2.
H bonds are broken into smaller intermediates. In addition, the detailed distribution and generation paths of the main products C2H4, H2, H2O, CH2O, and so on are depicted to construct the reaction mechanism of C17H34O2.
中文翻译:

ReaxFF-MD法棕榈酸甲酯的热解和燃烧反应机理
棕榈酸甲酯(C 17 H 34 O 2 )作为化石燃料的替代品,研究其热解和燃烧机理具有重要的科学和现实意义。本文采用ReaxFF-MD方法研究了C 17 H 34 O 2的反应机理和主要产物。分析了温度和O 2对C 17 H 34 O 2分解、主要产物和反应路径的影响,确定了其反应机理。结果表明,C 17 H 34 O 2热解和燃烧的反应活化能分别为201.76 kJ/mol和139.07 kJ/mol。在较高温度下,温度对C 17 H 34 O 2的影响大于O 2对C 17 H 34 O 2的影响。C 17 H 34 O 2初始分解的基本反应路径是C  C、C
C、C  O和C
O和C  H键断裂成更小的中间体。此外,还描绘了主要产物C 2 H 4、H 2、H 2 O、CH 2 O等的详细分布和生成路径,构建了C 17 H 34 O 2的反应机理。
H键断裂成更小的中间体。此外,还描绘了主要产物C 2 H 4、H 2、H 2 O、CH 2 O等的详细分布和生成路径,构建了C 17 H 34 O 2的反应机理。






































 京公网安备 11010802027423号
京公网安备 11010802027423号