当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pentlandite Compound-Anchored CuSCN as a Stable Electrocatalyst in Highly Alkaline Solutions
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-12-20 , DOI: 10.1021/acssuschemeng.3c04412 Nandapriya Manivelan 1 , Vaiyapuri Soundharrajan 2 , Jaekook Kim 3 , Kandasamy Prabakar 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-12-20 , DOI: 10.1021/acssuschemeng.3c04412 Nandapriya Manivelan 1 , Vaiyapuri Soundharrajan 2 , Jaekook Kim 3 , Kandasamy Prabakar 1
Affiliation
|
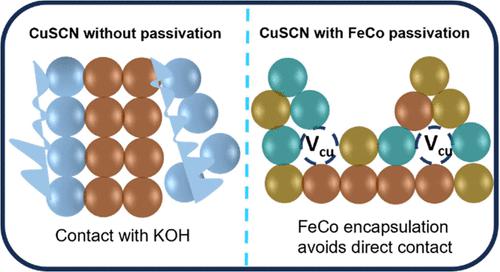
Copper thiocyanate (CuSCN) is an unexplored material for water-splitting reactions, and it is a well-known photocathode in dye-sensitized solar cells. For the first time, the electrochemical properties of CuSCN were investigated and used in overall water-splitting reactions. It is observed that when a negative potential is applied to CuSCN, it readily gets electrons from the circuit and gives them to the dissociated hydrogen radical (H+), which in turn facilitates more hydrogen adsorption sites (Had) and evolves hydrogen gas. Despite this advantage, chalcogenide materials (CuSCN) are not stable in alkaline solutions (pH = 14), where the surface gets easily oxidized in the KOH electrolyte by forming an S═O bond. Therefore, to prevent oxidation and material dissolution, we passivated the CuSCN surface with FeCo. Interestingly, the deposition of FeCo on CuSCN forms a pentlandite compound anchoring CuSCN, which suppresses the oxidation of the CuSCN surface in KOH solution by interatomic coupling of crystal systems and avoids direct contact of CuSCN with the electrolyte. Also, the inductively coupled plasma results show that the material dissolution concentration is greatly reduced from 2.67:26 to 0.37:3.4 (Cu:S) for FeCo-passivated CuSCN surfaces. The full cell constructed using CuSCN30 || FeCo/CuSCN30 as the cathode and the anode exhibits 1.70 V at 50 mA/cm2 and tremendous robustness at a high current density of 200 mA/cm2 for 50 h.
中文翻译:

镍黄铁矿化合物锚定的 CuSCN 在高碱性溶液中作为稳定的电催化剂
硫氰酸铜(CuSCN)是一种尚未开发的水分解反应材料,也是染料敏化太阳能电池中众所周知的光电阴极。首次研究了 CuSCN 的电化学性质并将其用于整个水分解反应。据观察,当对 CuSCN 施加负电位时,它很容易从电路中获取电子并将其提供给离解的氢自由基 (H + ),这反过来又促进更多的氢吸附位点 (H ad ) 并放出氢气。尽管有这样的优势,硫族化物材料 (CuSCN) 在碱性溶液 (pH = 14) 中并不稳定,表面很容易在 KOH 电解质中形成 S=O 键而被氧化。因此,为了防止氧化和材料溶解,我们用 FeCo 钝化了 CuSCN 表面。有趣的是,FeCo在CuSCN上的沉积形成了锚定CuSCN的镍黄铁矿化合物,该化合物通过晶系的原子间耦合抑制了KOH溶液中CuSCN表面的氧化,并避免了CuSCN与电解质的直接接触。此外,电感耦合等离子体结果表明,FeCo 钝化的 CuSCN 表面的材料溶解浓度从 2.67:26 大大降低至 0.37:3.4 (Cu:S)。使用 CuSCN30 构建的全电池 || FeCo/CuSCN30 作为阴极和阳极,在 50 mA/cm 2下表现出 1.70 V 的电压,并在 200 mA/cm 2的高电流密度下持续50 小时表现出巨大的鲁棒性。
更新日期:2023-12-20
中文翻译:

镍黄铁矿化合物锚定的 CuSCN 在高碱性溶液中作为稳定的电催化剂
硫氰酸铜(CuSCN)是一种尚未开发的水分解反应材料,也是染料敏化太阳能电池中众所周知的光电阴极。首次研究了 CuSCN 的电化学性质并将其用于整个水分解反应。据观察,当对 CuSCN 施加负电位时,它很容易从电路中获取电子并将其提供给离解的氢自由基 (H + ),这反过来又促进更多的氢吸附位点 (H ad ) 并放出氢气。尽管有这样的优势,硫族化物材料 (CuSCN) 在碱性溶液 (pH = 14) 中并不稳定,表面很容易在 KOH 电解质中形成 S=O 键而被氧化。因此,为了防止氧化和材料溶解,我们用 FeCo 钝化了 CuSCN 表面。有趣的是,FeCo在CuSCN上的沉积形成了锚定CuSCN的镍黄铁矿化合物,该化合物通过晶系的原子间耦合抑制了KOH溶液中CuSCN表面的氧化,并避免了CuSCN与电解质的直接接触。此外,电感耦合等离子体结果表明,FeCo 钝化的 CuSCN 表面的材料溶解浓度从 2.67:26 大大降低至 0.37:3.4 (Cu:S)。使用 CuSCN30 构建的全电池 || FeCo/CuSCN30 作为阴极和阳极,在 50 mA/cm 2下表现出 1.70 V 的电压,并在 200 mA/cm 2的高电流密度下持续50 小时表现出巨大的鲁棒性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号