当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting N2O Decomposition by Fabricating the Cs–O–Co Structure over Co3O4 with Single-Layer Atoms of Cs
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-12-21 , DOI: 10.1021/acs.est.3c06940
Yuanyu Gong 1 , Zhisong Liu 1 , Zihao Li 1 , Caixia Liu 2 , Naiqiang Yan 1 , Lei Ma 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-12-21 , DOI: 10.1021/acs.est.3c06940
Yuanyu Gong 1 , Zhisong Liu 1 , Zihao Li 1 , Caixia Liu 2 , Naiqiang Yan 1 , Lei Ma 1
Affiliation
|
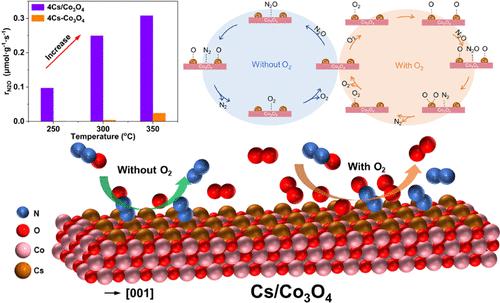
Developing effective catalysts for N2O decomposition at low temperatures is challenging. Herein, the Cs–O–Co structure, as the active species fabricated by single-layer atoms of Cs over pure Co3O4, originally exhibited great catalytic activity of N2O decomposition in simulated vehicle exhaust and flue gas from nitric acid plants. A similar catalytic performance was also observed for Na, K, and Rb alkali metals over Co3O4 catalysts for N2O decomposition, illustrating the prevalence of alkali-metal-promotion over Co3O4 in practical applications. The catalytic results indicated that the TOF of Co3O4 catalysts loaded by 4 wt% Cs was nearly 2 orders of magnitude higher than that of pure Co3O4 catalysts at 300 °C. Interestingly, the conversions of N2O decomposition over Co3O4 catalysts doped by the same Cs loadings were significantly inhibited. Characterization results indicated that the primary active Cs–O–Co structure was formed by highly orbital hybridization between the Cs 6s and the O 2p orbital over the supported Co3O4 catalysts, where Cs could donate electrons to Co3+ and produce much more Co2+. In contrast, the doped Co3O4 catalysts were dominated by Cs2O2 species; meanwhile, CsOH species was generated by adsorbed water vapor led to a significant decrease in catalytic activity. In situ DRIFTS, rigorous kinetics, and DFT results elaborated the reaction mechanism of N2O decomposition, where the direct decomposition of adsorbed N2O was the kinetically relevant step over supported catalysts in the absence of O2. Meanwhile, the assistance of adsorbed N2O decomposition by activated oxygen was observed as the kinetically relevant step in the presence of O2. The results may pave a promising path toward developing alkali-metal-promotion catalysts for efficient N2O decomposition.
中文翻译:

通过在 Co3O4 上用单层 Cs 原子制造 Cs-O-Co 结构来促进 N2O 分解
开发有效的低温 N 2 O 分解催化剂具有挑战性。其中,Cs-O-Co结构作为Cs单层原子在纯Co 3 O 4上制备的活性物种,最初在模拟汽车尾气和硝酸厂烟气中表现出良好的N 2 O分解催化活性。对于Na、K和Rb碱金属在Co 3 O 4催化剂上对于N 2 O分解也观察到类似的催化性能,这说明在实际应用中碱金属促进相对于Co 3 O 4的普遍性。催化结果表明,负载4 wt% Cs的Co 3 O 4催化剂在300 ℃下的TOF比纯Co 3 O 4催化剂高近2个数量级。有趣的是,在掺杂相同Cs负载量的Co 3 O 4催化剂上,N 2 O分解的转化率受到显着抑制。表征结果表明,初级活性Cs–O–Co结构是由Cs 6s和O 2p轨道在负载型Co 3 O 4催化剂上的高轨道杂化形成的,其中Cs可以向Co 3+提供电子并产生更多的电子。钴2+ 。相比之下,掺杂的Co 3 O 4催化剂主要由Cs 2 O 2物质组成;同时,吸附水蒸气产生CsOH物质,导致催化活性显着下降。 原位漂移、严格动力学和DFT结果详细阐述了N 2 O分解的反应机理,其中吸附的N 2 O的直接分解是在不存在O 2的情况下负载型催化剂上的动力学相关步骤。同时,观察到活性氧对吸附的N 2 O 分解的帮助是O 2存在下的动力学相关步骤。这些结果可能为开发用于高效 N 2 O 分解的碱金属促进催化剂铺平了一条有希望的道路。
更新日期:2023-12-21
中文翻译:

通过在 Co3O4 上用单层 Cs 原子制造 Cs-O-Co 结构来促进 N2O 分解
开发有效的低温 N 2 O 分解催化剂具有挑战性。其中,Cs-O-Co结构作为Cs单层原子在纯Co 3 O 4上制备的活性物种,最初在模拟汽车尾气和硝酸厂烟气中表现出良好的N 2 O分解催化活性。对于Na、K和Rb碱金属在Co 3 O 4催化剂上对于N 2 O分解也观察到类似的催化性能,这说明在实际应用中碱金属促进相对于Co 3 O 4的普遍性。催化结果表明,负载4 wt% Cs的Co 3 O 4催化剂在300 ℃下的TOF比纯Co 3 O 4催化剂高近2个数量级。有趣的是,在掺杂相同Cs负载量的Co 3 O 4催化剂上,N 2 O分解的转化率受到显着抑制。表征结果表明,初级活性Cs–O–Co结构是由Cs 6s和O 2p轨道在负载型Co 3 O 4催化剂上的高轨道杂化形成的,其中Cs可以向Co 3+提供电子并产生更多的电子。钴2+ 。相比之下,掺杂的Co 3 O 4催化剂主要由Cs 2 O 2物质组成;同时,吸附水蒸气产生CsOH物质,导致催化活性显着下降。 原位漂移、严格动力学和DFT结果详细阐述了N 2 O分解的反应机理,其中吸附的N 2 O的直接分解是在不存在O 2的情况下负载型催化剂上的动力学相关步骤。同时,观察到活性氧对吸附的N 2 O 分解的帮助是O 2存在下的动力学相关步骤。这些结果可能为开发用于高效 N 2 O 分解的碱金属促进催化剂铺平了一条有希望的道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号