当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Nature of Active Oxygen Species and Reaction Mechanism of Ethylene Epoxidation by Supported Ag/α-Al2O3 Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-12-19 , DOI: 10.1021/acscatal.3c04361
Tiancheng Pu 1 , Adhika Setiawan 2 , Alexandre C Foucher 3 , Mingyu Guo 1 , Jih-Mirn Jehng 1 , Minghui Zhu 4 , Michael E Ford 1 , Eric A Stach 3 , Srinivas Rangarajan 2 , Israel E Wachs 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-12-19 , DOI: 10.1021/acscatal.3c04361
Tiancheng Pu 1 , Adhika Setiawan 2 , Alexandre C Foucher 3 , Mingyu Guo 1 , Jih-Mirn Jehng 1 , Minghui Zhu 4 , Michael E Ford 1 , Eric A Stach 3 , Srinivas Rangarajan 2 , Israel E Wachs 1
Affiliation
|
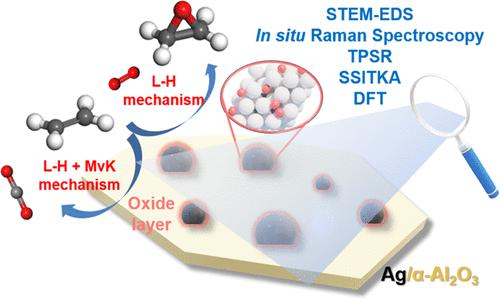
The oxygen species on Ag catalysts and reaction mechanisms for ethylene epoxidation and ethylene combustion continue to be debated in the literature despite decades of investigation. Fundamental details of ethylene oxidation by supported Ag/α-Al2O3 catalysts were revealed with the application of high-angle annular dark-field-scanning transmission electron microscopy-energy-dispersive X-ray spectroscopy (HAADF-STEM-EDS), in situ techniques (Raman, UV–vis, X-ray diffraction (XRD), HS-LEIS), chemical probes (C2H4-TPSR and C2H4 + O2-TPSR), and steady-state ethylene oxidation and SSITKA (16O2 → 18O2 switch) studies. The Ag nanoparticles are found to carry a considerable amount of oxygen after the reaction. Density functional theory (DFT) calculations indicate the oxidative reconstructed p(4 × 4)–O–Ag(111) surface is stable relative to metallic Ag(111) under the relevant reaction environment. Multiple configurations of reactive oxygen species are present, and their relevant concentrations depend on treatment conditions. Selective ethylene oxidation to EO proceeds with surface Ag4–O2* species (dioxygen species occupying an oxygen site on a p(4 × 4)–O–Ag(111) surface) only present after strong oxidation of Ag. These experimental findings are strongly supported by the associated DFT calculations. Ethylene epoxidation proceeds via a Langmuir–Hinshelwood mechanism, and ethylene combustion proceeds via combined Langmuir–Hinshelwood (predominant) and Mars–van Krevelen (minor) mechanisms.
中文翻译:

揭示负载型Ag/α-Al2O3催化剂活性氧的性质及乙烯环氧化反应机理
尽管经过了数十年的研究,银催化剂上的氧物种以及乙烯环氧化和乙烯燃烧的反应机制在文献中仍然存在争议。应用高角度环形暗场扫描透射电子显微镜-能量色散X射线光谱仪(HAADF-STEM-EDS)揭示了负载型Ag/α-Al 2 O 3催化剂乙烯氧化的基本细节,原位技术(拉曼、UV-vis、X 射线衍射 (XRD)、HS-LEIS)、化学探针(C 2 H 4 -TPSR 和 C 2 H 4 + O 2 -TPSR)和稳态乙烯氧化和SSITKA( 16 O 2 → 18 O 2转换)研究。反应后发现银纳米颗粒携带大量氧气。密度泛函理论(DFT)计算表明,在相关反应环境下,氧化重建的p(4×4)–O–Ag(111)表面相对于金属Ag(111)表面是稳定的。活性氧的多种构型存在,并且它们的相关浓度取决于处理条件。选择性乙烯氧化为 EO 时,表面 Ag 4 –O 2 * 物质(双氧物质占据 ap(4 × 4)–O–Ag(111) 表面上的氧位点)仅在 Ag 强氧化后出现。这些实验结果得到了相关 DFT 计算的有力支持。乙烯环氧化通过 Langmuir-Hinshelwood 机制进行,乙烯燃烧通过 Langmuir-Hinshelwood(主要)和 Mars-van Krevelen(次要)机制进行。
更新日期:2023-12-19
中文翻译:

揭示负载型Ag/α-Al2O3催化剂活性氧的性质及乙烯环氧化反应机理
尽管经过了数十年的研究,银催化剂上的氧物种以及乙烯环氧化和乙烯燃烧的反应机制在文献中仍然存在争议。应用高角度环形暗场扫描透射电子显微镜-能量色散X射线光谱仪(HAADF-STEM-EDS)揭示了负载型Ag/α-Al 2 O 3催化剂乙烯氧化的基本细节,原位技术(拉曼、UV-vis、X 射线衍射 (XRD)、HS-LEIS)、化学探针(C 2 H 4 -TPSR 和 C 2 H 4 + O 2 -TPSR)和稳态乙烯氧化和SSITKA( 16 O 2 → 18 O 2转换)研究。反应后发现银纳米颗粒携带大量氧气。密度泛函理论(DFT)计算表明,在相关反应环境下,氧化重建的p(4×4)–O–Ag(111)表面相对于金属Ag(111)表面是稳定的。活性氧的多种构型存在,并且它们的相关浓度取决于处理条件。选择性乙烯氧化为 EO 时,表面 Ag 4 –O 2 * 物质(双氧物质占据 ap(4 × 4)–O–Ag(111) 表面上的氧位点)仅在 Ag 强氧化后出现。这些实验结果得到了相关 DFT 计算的有力支持。乙烯环氧化通过 Langmuir-Hinshelwood 机制进行,乙烯燃烧通过 Langmuir-Hinshelwood(主要)和 Mars-van Krevelen(次要)机制进行。

































 京公网安备 11010802027423号
京公网安备 11010802027423号