Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2023-12-20 , DOI: 10.1016/j.enzmictec.2023.110391
David Gercke 1 , Florian Lenz 1 , Joachim Jose 1
|
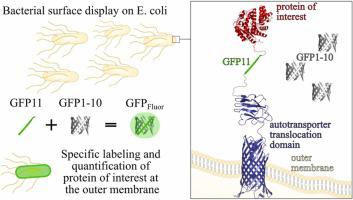
The split-GFP system is a versatile tool with numerous applications, but it has been underutilized for the labeling of heterologous surface-displayed proteins. By inserting the 16 amino acid sequence of the GFP11-tag between a protein of interest and an autotransporter protein, it is possible to present a protein at the outer membrane of gram-negative bacteria and to fluorescently label it by complementation with externally added GFP1–10. The labeled cells could be clearly discerned from cells without the protein of interest using flow cytometry and the insertion of the GFP11-tag caused no significant alteration of the catalytic activity for the tested model enzyme CsBglA. Furthermore, the amount of the protein of interest on the cells could be quantified by comparing the green fluorescence resulting from the complementation to that of standards with known concentrations. This allows a precise characterization of whole-cell biocatalysts, which is difficult with existing methods. The split-GFP complementation approach was shown to be specific, in a similar manner as commercial antibodies. It is cost-efficient, minimizes the possibility of adverse effects on protein expression or solubility, and can be performed at high throughput.
中文翻译:

细菌细胞表面的 split-GFP 互补,用于无抗体标记和异源蛋白展示定量
split-GFP 系统是一种具有多种应用的多功能工具,但在异源表面展示蛋白的标记方面尚未得到充分利用。通过在感兴趣的蛋白质和自转运蛋白之间插入 GFP11 标签的 16 个氨基酸序列,可以在革兰氏阴性细菌的外膜上呈现蛋白质,并通过与外部添加的 GFP1 互补来荧光标记它 - 10.使用流式细胞术可以清楚地区分标记的细胞与不含目的蛋白的细胞,并且 GFP11 标签的插入不会导致测试模型酶 CsBglA 的催化活性发生显着改变。此外,通过将互补产生的绿色荧光与已知浓度的标准品的绿色荧光进行比较,可以对细胞上感兴趣的蛋白质的量进行定量。这使得对全细胞生物催化剂进行精确表征成为可能,而这对于现有方法来说是困难的。分裂-GFP 互补方法被证明是特异性的,其方式与商业抗体类似。它具有成本效益,最大限度地减少对蛋白质表达或溶解度产生不利影响的可能性,并且可以高通量进行。

































 京公网安备 11010802027423号
京公网安备 11010802027423号