当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-Sulfonyl amidine polypeptides: new polymeric biomaterials with conformation transition responsive to tumor acidity
Chemical Science ( IF 7.6 ) Pub Date : 2023-12-20 , DOI: 10.1039/d3sc05504c Xiang Xu 1 , Jinjuan Ma 2 , Aiguo Wang 2 , Nan Zheng 1, 3
Chemical Science ( IF 7.6 ) Pub Date : 2023-12-20 , DOI: 10.1039/d3sc05504c Xiang Xu 1 , Jinjuan Ma 2 , Aiguo Wang 2 , Nan Zheng 1, 3
Affiliation
|
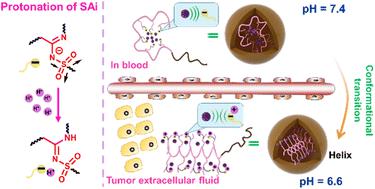
Manipulation of pH responsiveness is a frequently employed tactic in the formulation of trigger-responsive nanomaterials. It offers an avenue for “smart” designs capitalizing on distinctive pH gradients across diverse tissues and intracellular compartments. However, an overwhelming majority of documented functional groups (>80%) exhibit responsiveness solely to the heightened acidic milieu of intracellular pH (about 4.5–5.5). This scenario diverges markedly from the moderately acidic extracellular pH (∼6.8) characteristic of tumor microenvironments. Consequently, systems predicated upon intracellular pH responsiveness are unlikely to confer discernible advantages concerning targeted penetration and cellular uptake at tumor sites. In this study, we elucidated the extracellular pH responsiveness intrinsic to N-sulfonyl amidine (SAi), delineating a method to synthesize an array of SAi-bearing polypeptides (SAi-polypeptides). Notably, we demonstrated the pH-dependent modulation of SAi-polypeptide conformations, made possible by the protonation/deprotonation equilibrium of SAi in response to minute fluctuations in pH from physiological conditions to the extracellular milieu of tumors. This dynamic pH-triggered transition of SAi-polypeptides from negatively charged to neutrally charged side chains at the pH outside tumor cells (∼6.8) facilitated a transition from coil to helix conformations, concomitant with the induction of cellular internalization upon arrival at tumor sites. Furthermore, the progressive acidification of the intracellular environment expedited drug release, culminating in significantly enhanced site-specific chemotherapeutic efficacy compared with free-drug counterparts. The distinct pH-responsive attributes of SAi could aid the design of tumor acidity-responsive applications, thereby furnishing invaluable insights into the realm of smart material design.
中文翻译:

N-磺酰脒多肽:具有响应肿瘤酸度的构象转变的新型聚合物生物材料
操纵 pH 响应性是触发响应纳米材料配制中经常采用的策略。它为“智能”设计提供了一条途径,利用不同组织和细胞内区室的独特 pH 梯度。然而,绝大多数已记录的官能团(>80%)仅对细胞内 pH 值升高的酸性环境(约 4.5-5.5)表现出响应性。这种情况与肿瘤微环境的中等酸性细胞外 pH (∼6.8) 特征明显不同。因此,基于细胞内 pH 响应的系统不太可能在肿瘤部位的靶向渗透和细胞摄取方面提供明显的优势。在这项研究中,我们阐明了N-磺酰脒 (SAi) 固有的细胞外 pH 响应性,描述了合成一系列 SAi 多肽(SAi 多肽)的方法。值得注意的是,我们证明了 SAi 多肽构象的 pH 依赖性调节,这是通过 SAi 的质子化/去质子化平衡响应从生理条件到肿瘤细胞外环境的 pH 微小波动而实现的。在肿瘤细胞外的 pH 值 (~6.8) 下,SAi 多肽由 pH 值触发的动态从带负电的侧链转变为带中性电荷的侧链,促进了从卷曲构象到螺旋构象的转变,同时在到达肿瘤部位时诱导细胞内化。此外,细胞内环境的逐渐酸化加速了药物释放,最终与游离药物相比显着增强了位点特异性化疗功效。 SAi 独特的 pH 响应属性可以帮助肿瘤酸度响应应用的设计,从而为智能材料设计领域提供宝贵的见解。
更新日期:2023-12-20
中文翻译:

N-磺酰脒多肽:具有响应肿瘤酸度的构象转变的新型聚合物生物材料
操纵 pH 响应性是触发响应纳米材料配制中经常采用的策略。它为“智能”设计提供了一条途径,利用不同组织和细胞内区室的独特 pH 梯度。然而,绝大多数已记录的官能团(>80%)仅对细胞内 pH 值升高的酸性环境(约 4.5-5.5)表现出响应性。这种情况与肿瘤微环境的中等酸性细胞外 pH (∼6.8) 特征明显不同。因此,基于细胞内 pH 响应的系统不太可能在肿瘤部位的靶向渗透和细胞摄取方面提供明显的优势。在这项研究中,我们阐明了N-磺酰脒 (SAi) 固有的细胞外 pH 响应性,描述了合成一系列 SAi 多肽(SAi 多肽)的方法。值得注意的是,我们证明了 SAi 多肽构象的 pH 依赖性调节,这是通过 SAi 的质子化/去质子化平衡响应从生理条件到肿瘤细胞外环境的 pH 微小波动而实现的。在肿瘤细胞外的 pH 值 (~6.8) 下,SAi 多肽由 pH 值触发的动态从带负电的侧链转变为带中性电荷的侧链,促进了从卷曲构象到螺旋构象的转变,同时在到达肿瘤部位时诱导细胞内化。此外,细胞内环境的逐渐酸化加速了药物释放,最终与游离药物相比显着增强了位点特异性化疗功效。 SAi 独特的 pH 响应属性可以帮助肿瘤酸度响应应用的设计,从而为智能材料设计领域提供宝贵的见解。





































 京公网安备 11010802027423号
京公网安备 11010802027423号