当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemiluminescence of a Firefly Luciferin Analogue Reveals that Formation of the Key Intermediate Responsible for Excited State Generation Occurs on a Fully Concerted Step
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-12-19 , DOI: 10.1021/acs.joc.3c02079 Diego Ulysses Melo 1 , Henrique Bergonzini de Lima 1 , Roberta Albino Reis 1 , Andreia Boaro 1 , Alexander Garreta Gonçalves Costa Pinto 1 , Luiz Francisco Monteiro Leite Ciscato 1 , Paula Homem-de-Mello 1 , Fernando Heering Bartoloni 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-12-19 , DOI: 10.1021/acs.joc.3c02079 Diego Ulysses Melo 1 , Henrique Bergonzini de Lima 1 , Roberta Albino Reis 1 , Andreia Boaro 1 , Alexander Garreta Gonçalves Costa Pinto 1 , Luiz Francisco Monteiro Leite Ciscato 1 , Paula Homem-de-Mello 1 , Fernando Heering Bartoloni 1
Affiliation
|
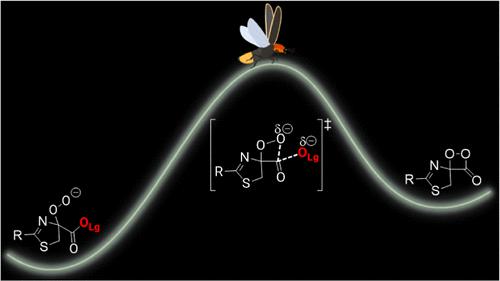
The chemiluminescence (CL) reaction of eight different 2-(4-hydroxyphenyl)-4,5-dihydrothiazole-4-carboxylate esters with an organic superbase and oxygen was investigated through a kinetic and computational study. These esters are all analogues to the luciferin substrate involved in efficient firefly bioluminescence. The kinetic data obtained from CL emission and light absorption assays were used in the context of linear free energy relationships (LFER); we obtained the Hammett reaction constant ρ = +1.62 ± 0.09 and the Brønsted constant βlg = −0.39 ± 0.04. These observations from LFER, together with activation parameters obtained from Arrhenius plots, suggest that the formation of the high-energy intermediate (HEI) 1,2-dioxetanone occurs via a concerted mechanism during the rate-determining step of the reaction. Calculations performed using density functional theory support a late transition state for HEI formation within the reaction mechanism pathway, which was described considering geometric parameters, Wiberg bond indices from natural bond order analysis, and the atomic charges derived from the electrostatic potential.
中文翻译:

萤火虫荧光素类似物的化学发光揭示了负责激发态生成的关键中间体的形成是在完全一致的步骤中发生的
通过动力学和计算研究研究了八种不同的 2-(4-羟基苯基)-4,5-二氢噻唑-4-甲酸酯与有机超强碱和氧气的化学发光 (CL) 反应。这些酯都是参与有效萤火虫生物发光的荧光素底物的类似物。从 CL 发射和光吸收测定中获得的动力学数据用于线性自由能关系 (LFER);我们得到哈米特反应常数 ρ = +1.62 ± 0.09 和布朗斯台德常数 β lg = -0.39 ± 0.04。LFER 的这些观察结果以及从阿累尼乌斯图获得的活化参数表明,高能中间体 (HEI) 1,2-二氧杂环丁酮的形成是在反应的速率决定步骤期间通过协调机制发生的。使用密度泛函理论进行的计算支持反应机制路径内 HEI 形成的后期过渡态,该状态是考虑几何参数、自然键序分析的 Wiberg 键指数以及源自静电势的原子电荷来描述的。
更新日期:2023-12-19
中文翻译:

萤火虫荧光素类似物的化学发光揭示了负责激发态生成的关键中间体的形成是在完全一致的步骤中发生的
通过动力学和计算研究研究了八种不同的 2-(4-羟基苯基)-4,5-二氢噻唑-4-甲酸酯与有机超强碱和氧气的化学发光 (CL) 反应。这些酯都是参与有效萤火虫生物发光的荧光素底物的类似物。从 CL 发射和光吸收测定中获得的动力学数据用于线性自由能关系 (LFER);我们得到哈米特反应常数 ρ = +1.62 ± 0.09 和布朗斯台德常数 β lg = -0.39 ± 0.04。LFER 的这些观察结果以及从阿累尼乌斯图获得的活化参数表明,高能中间体 (HEI) 1,2-二氧杂环丁酮的形成是在反应的速率决定步骤期间通过协调机制发生的。使用密度泛函理论进行的计算支持反应机制路径内 HEI 形成的后期过渡态,该状态是考虑几何参数、自然键序分析的 Wiberg 键指数以及源自静电势的原子电荷来描述的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号