Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-12-18 , DOI: 10.1016/j.actbio.2023.12.020 Na Song 1 , Zhe Sun 2 , Bo Wang 3 , Xin Liu 4 , Binbin Hu 4 , Ninglin Chen 5 , Sihe Zhang 3 , Zhilin Yu 4
|
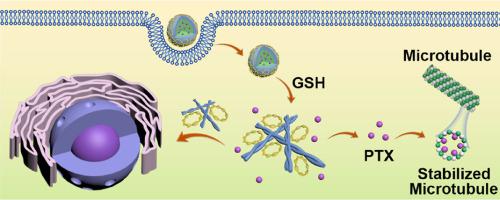
Suicide gene therapy is a promising therapeutic model for ovarian cancer (OC), while suffering from poor gene delivery and limited therapeutic efficacy. To address this concern, here we reported the GSH-responsive morphology-transformable enantiomeric peptide assemblies as delivering vehicles for suicide genes and co-delivery of paclitaxel (PTX). Connecting a lipid-like amphiphile and a hydrophilic arginine segment through disulfide bonds led to the enantiomeric peptides. The enantiomeric peptide assemblies are able to simultaneously uptake plasmid DNA (pDNA) and PTX based on electrostatic and hydrophobic interactions. The resulting co-assemblies underwent GSH-responsive disulfide cleavage and thereby promoting their assembly from nanoparticles to nanofibers, leading to the co-release of pDNA and PTX. Cellular and animal studies confirmed the co-delivery of pDNA and PTX into OC cells and the cell apoptosis by the enantiomeric peptides. In addition, in vitro and in vivo experiments supported the advanced uptake and cytotoxicity for L-type peptide vehicles by OC cells, and their great potential for OC-imaging, growth-inhibition and apoptosis-induction compared to D-counterpart. Our results demonstrate that the GSH-responsive morphology-transformable chiral peptide assemblies accurately and simultaneously release suicide genes and chemodrugs at tumor sites, thus providing a new strategy for the development of delivering vehicles for suicide gene and establishment of new therapeutic models for ovarian cancer.
Statement of significance
Appropriate delivery carriers are essential for the clinical translation of cancer gene therapy, including the emerging suicide gene therapy. By combining the advantages of morphological transformable vehicles with the chirality peptides towards their bioactivity, we developed the GSH-responsive morphology-transformable enantiomeric peptide assemblies as delivering vehicles for suicide genes and co-delivery of paclitaxel. The GSH-responsive assembly of the enantiomeric peptides allows for precise release of plasmid DNA and paclitaxel in cancer cells, and promotes the formation of nanofibrils that facilitate gene entering nuclei for transfection. The enantiomeric peptide-based vehicles show the chirality-dependent capability for inducing cell apoptosis and inhibiting tumor growth. Our findings demonstrate a new strategy for developing therapeutic models for ovarian cancer.
中文翻译:

通过形态适应性对映体肽组装体递送自杀基因用于卵巢癌联合治疗
自杀基因疗法是卵巢癌(OC)的一种有前途的治疗模式,但其基因传递较差且治疗效果有限。为了解决这个问题,我们在这里报道了 GSH 响应形态可转化的对映体肽组装体作为自杀基因的传递载体和紫杉醇 (PTX) 的共同传递。通过二硫键连接类脂两亲物和亲水性精氨酸片段形成对映体肽。对映体肽组装体能够基于静电和疏水相互作用同时摄取质粒 DNA (pDNA) 和 PTX。由此产生的共组装体经历了 GSH 响应性二硫键裂解,从而促进它们从纳米颗粒组装成纳米纤维,导致 pDNA 和 PTX 的共同释放。细胞和动物研究证实了 pDNA 和 PTX 共同递送到 OC 细胞中,并且对映体肽导致细胞凋亡。此外,体外和体内实验支持 OC 细胞对 L 型肽载体的先进摄取和细胞毒性,以及与 D 对应物相比,它们在 OC 成像、生长抑制和凋亡诱导方面的巨大潜力。我们的研究结果表明,GSH响应性形态可转化的手性肽组装体能够在肿瘤位点准确、同时释放自杀基因和化疗药物,从而为开发自杀基因递送载体和建立卵巢癌新治疗模型提供了新策略。
重要性声明
合适的递送载体对于癌症基因治疗(包括新兴的自杀基因治疗)的临床转化至关重要。通过将形态可转化载体的优点与手性肽的生物活性相结合,我们开发了 GSH 响应形态可转化对映体肽组装体,作为自杀基因的递送载体和紫杉醇的共同递送。对映体肽的 GSH 响应性组装可以在癌细胞中精确释放质粒 DNA 和紫杉醇,并促进纳米原纤维的形成,从而促进基因进入细胞核进行转染。基于对映体肽的载体显示出诱导细胞凋亡和抑制肿瘤生长的手性依赖性能力。我们的研究结果展示了开发卵巢癌治疗模型的新策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号