当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroboration Reduction of CO2 Catalyzed by a Doubly Reduced Arylborane: DFT Insight into Double Active-Site and CO2 Self-Promoting Single Active-Site Mechanisms and Counterion Effects
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-12-19 , DOI: 10.1021/acscatal.3c04386 Lin Zhang 1 , Ming Lei 2 , Zexing Cao 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-12-19 , DOI: 10.1021/acscatal.3c04386 Lin Zhang 1 , Ming Lei 2 , Zexing Cao 1
Affiliation
|
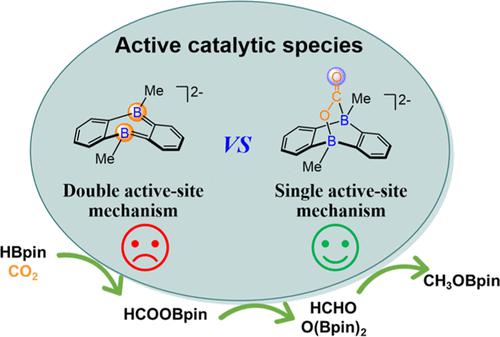
Hydroboration reduction of CO2 by HBpin catalyzed by ambiphilic boron centers, a doubly reduced 9,10-dimethyl-9,10-dihydro-9,10-diboraanthracene [DBA-Me2]2– (A0) and its metal salts (MA0) reported by Wagner, M. ( Angew. Chem., Int. Ed. 2018, 57, 16491–16495), has been explored by density functional theory (DFT) calculations. Unexpectedly, the cycloadduct (A1a) from the barrier-free activation of CO2 and bridging across the two B atoms of A0 may serve as a highly active catalyst to boost the CO2 hydroboration reduction through a single active-site mechanism. The catalytic hydroboration of CO2 by dianion A0 and its metal salts Mn-[DBA-Me2] (n = 2 for M = Li+, Na+, K+; and n = 1 for M = Mg2+, Ca2+) follows the double active-site mechanism, where the diboron site acts as the active center. Generally, the CO2 hydroboration reduction by HBpin may take place through three independent and cascade subcycles, leading to primary HCOOBpin, secondary HCHO, and reduction product CH3OBpin, respectively. Counter cations and THF solvent molecules remarkably influence the activation of HBpin and H2 by A0, but the relative energetics of the overall hydroboration process mediated by A1a is less changed. The present results show that the doubly reduced arylborane and its CO2 adduct, as well as their metal salts are quite promising for the hydroboration reduction of CO2.
中文翻译:

双还原芳基硼烷催化 CO2 硼氢化还原:DFT 洞察双活性位点和 CO2 自促进单活性位点机制和抗衡离子效应
两亲性硼中心、双重还原的 9,10-二甲基-9,10-二氢-9,10-二硼蒽 [DBA-Me 2 ] 2– ( A0 ) 及其金属盐 ( M ) 催化 HBpin 硼氢化还原 CO 2 A0 ) 报告者瓦格纳,M. ( Angew. Chem., Int. Ed. 2018 , 57 , 16491–16495) 已通过密度泛函理论 (DFT) 计算进行了探索。出乎意料的是,CO 2的无障碍活化和桥接A0的两个B原子形成的环加合物( A1a )可以作为高活性催化剂,通过单一活性位点机制促进CO 2 硼氢化还原。二阴离子A0及其金属盐Mn - [DBA-Me 2 ] ( n = 2 for M = Li + , Na + , K + ; and n = 1 for M = Mg 2+ , Ca n = 1 for M = Mg 2+ , Ca 2+ )遵循双活性位点机制,其中二硼位点充当活性中心。一般而言,HBpin的CO 2硼氢化还原可通过三个独立且级联的子循环进行,分别产生初级HCOOBpin、次级HCHO和还原产物CH 3 OBpin。抗衡阳离子和THF溶剂分子显着影响A0对HBpin和H 2的活化,但由A1a介导的整个硼氢化过程的相对能量变化较小。目前的结果表明,双还原芳基硼烷及其CO 2加合物以及它们的金属盐在CO 2的硼氢化还原方面非常有前景。
更新日期:2023-12-19
中文翻译:

双还原芳基硼烷催化 CO2 硼氢化还原:DFT 洞察双活性位点和 CO2 自促进单活性位点机制和抗衡离子效应
两亲性硼中心、双重还原的 9,10-二甲基-9,10-二氢-9,10-二硼蒽 [DBA-Me 2 ] 2– ( A0 ) 及其金属盐 ( M ) 催化 HBpin 硼氢化还原 CO 2 A0 ) 报告者































 京公网安备 11010802027423号
京公网安备 11010802027423号