当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of Spiro[chromene-2,4′-piperidine]s as Potent, Selective, and Gq-Biased 5-HT2C Receptor Partial Agonists
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2023-12-18 , DOI: 10.1021/acsmedchemlett.3c00454
Guangqian Jiang 1 , Bingjie Zhang 2, 3 , Xiaoya Zhang 1 , Fan Chen 1 , Wangzhi Qin 1 , Jing-Lei Chen 1 , Sheng Tian 1 , Wenqing Shui 2, 3 , Na Ye 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2023-12-18 , DOI: 10.1021/acsmedchemlett.3c00454
Guangqian Jiang 1 , Bingjie Zhang 2, 3 , Xiaoya Zhang 1 , Fan Chen 1 , Wangzhi Qin 1 , Jing-Lei Chen 1 , Sheng Tian 1 , Wenqing Shui 2, 3 , Na Ye 1
Affiliation
|
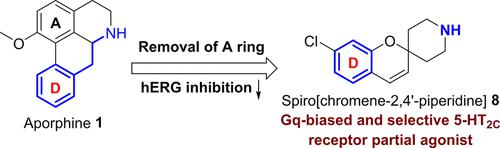
A series of spiropiperidines was designed and synthesized by structural modifications based on our previous lead compound 1 and evaluated with cellular signaling assays for the discovery of 5-HT2C receptor (5-HT2CR) selective agonists with a Gq bias. Structure–activity relationship (SAR) studies of spiropiperidines uncovered spiro[chromene-2,4′-piperidine]s as a novel chemotype of 5-HT2CR selective agonists. Among this new series, the 7-chloro analogue 8 was identified as the most potent and selective 5-HT2CR partial agonist (Emax = 71.09%) with an EC50 value of 121.5 nM and no observed activity toward 5-HT2AR or 5-HT2BR. Moreover, compound 8 exhibited no recruitment activity for β-arrestin and showed low inhibition of hERG at 10 μM. These findings may pave the way to develop more potent Gq-biased 5-HT2CR partial agonists as useful pharmacological tool compounds or potential drug candidates.
中文翻译:

螺 [铬-2,4′-哌啶] 鉴定为有效、选择性和 gq 偏倚的 5-HT2C 受体部分激动剂
基于我们之前的先导化合物 1,通过结构修饰设计和合成了一系列螺哌啶,并用细胞信号测定评估了发现具有 Gq 偏倚的 5-HT2C 受体 (5-HT2CR) 选择性激动剂。螺哌啶的构效关系 (SAR) 研究发现螺 [铬-2,4′-哌啶] 是 5-HT2CR 选择性激动剂的新型化学型。在这个新系列中,7-氯类似物 8 被确定为最有效和选择性的 5-HT2CR 部分激动剂 (Emax = 71.09%),EC50 值为 121.5 nM,未观察到对 5-HT2AR 或 5-HT2BR 的活性。此外,化合物 8 对 β-arrestin 没有募集活性,并且在 10 μM 时对 hERG 的抑制作用较低。这些发现可能为开发更有效的 Gq 偏倚 5-HT2CR 部分激动剂铺平道路,作为有用的药理学工具化合物或潜在的候选药物。
更新日期:2023-12-18
中文翻译:

螺 [铬-2,4′-哌啶] 鉴定为有效、选择性和 gq 偏倚的 5-HT2C 受体部分激动剂
基于我们之前的先导化合物 1,通过结构修饰设计和合成了一系列螺哌啶,并用细胞信号测定评估了发现具有 Gq 偏倚的 5-HT2C 受体 (5-HT2CR) 选择性激动剂。螺哌啶的构效关系 (SAR) 研究发现螺 [铬-2,4′-哌啶] 是 5-HT2CR 选择性激动剂的新型化学型。在这个新系列中,7-氯类似物 8 被确定为最有效和选择性的 5-HT2CR 部分激动剂 (Emax = 71.09%),EC50 值为 121.5 nM,未观察到对 5-HT2AR 或 5-HT2BR 的活性。此外,化合物 8 对 β-arrestin 没有募集活性,并且在 10 μM 时对 hERG 的抑制作用较低。这些发现可能为开发更有效的 Gq 偏倚 5-HT2CR 部分激动剂铺平道路,作为有用的药理学工具化合物或潜在的候选药物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号