当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-pot two-stage biocatalytic upgrading of biomass-derived aldehydes to optically active β-amino alcohols via sequential hydroxymethylation and asymmetric reduction amination
Green Chemistry ( IF 9.3 ) Pub Date : 2023-12-18 , DOI: 10.1039/d3gc04287a Yin-Hua Suo 1 , Jing-Qi Zhang 1 , Ning Qi 1 , Shuang-Ping Huang 1 , Hang Gao 1 , Li-Li Gao 2 , Chao-Feng Zhang 1 , Yu-Cai He 3 , Jian-Dong Zhang 1
Green Chemistry ( IF 9.3 ) Pub Date : 2023-12-18 , DOI: 10.1039/d3gc04287a Yin-Hua Suo 1 , Jing-Qi Zhang 1 , Ning Qi 1 , Shuang-Ping Huang 1 , Hang Gao 1 , Li-Li Gao 2 , Chao-Feng Zhang 1 , Yu-Cai He 3 , Jian-Dong Zhang 1
Affiliation
|
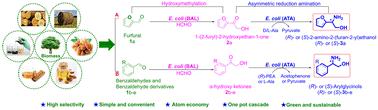
Chiral β-amino alcohols are crucial building blocks in the synthesis of pharmaceutically active molecules and natural products. Developing green and sustainable methods for synthesizing chiral β-amino alcohols from renewable feedstocks continues to pose a significant challenge. Herein, we proposed a simple one-pot two-stage artificial cascade biocatalysis system for highly efficient conversion of renewable biomass-derived aldehydes into chiral β-amino alcohols with good yields and excellent enantioselectivity via sequential hydroxymethylation and asymmetric reduction amination. The initial stage involves converting aldehydes into α-hydroxy ketones by providing formaldehyde through benzaldehyde lyase-catalyzed hydroxymethylation. The subsequent stage is to convert α-hydroxy ketones into chiral β-amino alcohols through amine transaminase-catalyzed asymmetric reduction amination. Combining the two different types of reactions with two different enzymes, biomass-derived furfural 1a could be easily converted into (R)-2-amino-2-(furan-2-yl)ethanol ((R)-3a) and (S)-2-amino-2-(furan-2-yl)ethanol ((S)-3a) with up to 95% and 92% conversions and >99% ee, respectively. In addition, with this cascade biocatalysis, several enantiopure arylglycinols 3b–e were synthesized (39.4–99.0% conversions and >99% ee) from the biomass-derived benzaldehydes (1b–c) and benzaldehyde derivatives (1d–e). Furthermore, biotransformation on a preparative scale gave (S)-3a and (R)-3a in good yields (64.6% and 60.4%) and excellent ee (>99%). This study presents an eco-friendly and sustainable approach for synthesizing chiral β-amino alcohols using biomass-derived aldehydes.
中文翻译:

通过连续羟甲基化和不对称还原胺化将生物质衍生的醛一锅两阶段生物催化升级为光学活性β-氨基醇
手性 β-氨基醇是合成药物活性分子和天然产物的重要组成部分。开发从可再生原料合成手性 β-氨基醇的绿色和可持续方法仍然是一个重大挑战。在此,我们提出了一种简单的一锅两级人工级联生物催化系统,通过顺序羟甲基化和不对称还原胺化,将可再生生物质衍生的醛高效转化为手性β-氨基醇,具有良好的收率和优异的对映选择性。初始阶段涉及通过苯甲醛裂解酶催化的羟甲基化提供甲醛,将醛转化为 α-羟基酮。下一步是通过胺转氨酶催化的不对称还原胺化将α-羟基酮转化为手性β-氨基醇。将两种不同类型的反应与两种不同的酶相结合,生物质衍生的糠醛1a可以很容易地转化为( R )-2-氨基-2-(呋喃-2-基)乙醇(( R ) -3a )和( S ) )-2-氨基-2-(呋喃-2-基)乙醇(( S ) -3a ),转化率分别高达95%和92%,ee>99%。此外,通过这种级联生物催化,从生物质衍生的苯甲醛 ( 1b–c ) 和苯甲醛衍生物 ( 1d–e ) 合成了几种对映体纯芳基甘氨醇3b–e (转化率 39.4–99.0%, ee > 99% )。此外,制备规模的生物转化以良好的收率(64.6%和60.4%)和优异的ee(> 99%)产生( S)-3a和(R)-3a 。这项研究提出了一种利用生物质衍生的醛合成手性 β-氨基醇的环保且可持续的方法。
更新日期:2023-12-21
中文翻译:

通过连续羟甲基化和不对称还原胺化将生物质衍生的醛一锅两阶段生物催化升级为光学活性β-氨基醇
手性 β-氨基醇是合成药物活性分子和天然产物的重要组成部分。开发从可再生原料合成手性 β-氨基醇的绿色和可持续方法仍然是一个重大挑战。在此,我们提出了一种简单的一锅两级人工级联生物催化系统,通过顺序羟甲基化和不对称还原胺化,将可再生生物质衍生的醛高效转化为手性β-氨基醇,具有良好的收率和优异的对映选择性。初始阶段涉及通过苯甲醛裂解酶催化的羟甲基化提供甲醛,将醛转化为 α-羟基酮。下一步是通过胺转氨酶催化的不对称还原胺化将α-羟基酮转化为手性β-氨基醇。将两种不同类型的反应与两种不同的酶相结合,生物质衍生的糠醛1a可以很容易地转化为( R )-2-氨基-2-(呋喃-2-基)乙醇(( R ) -3a )和( S ) )-2-氨基-2-(呋喃-2-基)乙醇(( S ) -3a ),转化率分别高达95%和92%,ee>99%。此外,通过这种级联生物催化,从生物质衍生的苯甲醛 ( 1b–c ) 和苯甲醛衍生物 ( 1d–e ) 合成了几种对映体纯芳基甘氨醇3b–e (转化率 39.4–99.0%, ee > 99% )。此外,制备规模的生物转化以良好的收率(64.6%和60.4%)和优异的ee(> 99%)产生( S)-3a和(R)-3a 。这项研究提出了一种利用生物质衍生的醛合成手性 β-氨基醇的环保且可持续的方法。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号