当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding of interfacial molecular interactions and inner-sphere reaction mechanism in heterogeneous Fenton-like catalysis on Mn-N4 site
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-12-17 , DOI: 10.1016/j.apcatb.2023.123619
Pijun Duan , Mingxue Li , Xing Xu , Qinyan Yue , Yue Gao , Baoyu Gao
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-12-17 , DOI: 10.1016/j.apcatb.2023.123619
Pijun Duan , Mingxue Li , Xing Xu , Qinyan Yue , Yue Gao , Baoyu Gao
|
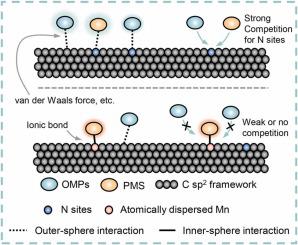
This study investigated interface molecular interactions between atomically-dispersed active sites and reactants in Fenton-like catalysis for deep understanding catalytic mechanism and dynamics. N-doped carbon nanotubes (NCNTs), atomically-dispersed Mn onto NCNTs (Mn-NCNT) and peroxymonosulfate (PMS) were applied as the research platforms. PMS adsorption on atomically-dispersed Mn sites was found to perform an inner-sphere interaction rather than an outer-sphere interaction on NCNT. The inner-sphere interaction leads to efficient electron transfer and robust PMS adsorption in the presence of organic pollutants, therefore high decontamination efficiency in Fenton-like catalysis. Theoretical calculation, X-ray electron spectrum, X-ray absorption spectrum indicated chemical bonds, specifically ionic bonds, were formed between Mn-N moieties and PMS. In contrast, the N-doped surface adsorbed PMS via van der Waals interactions. These findings provide a deep understanding of solid/liquid interface processes and additionally gives a new perspective on the superiority of single atom catalysts.
中文翻译:

理解Mn-N4位点多相芬顿催化中的界面分子相互作用和内层反应机制
本研究研究了类芬顿催化中原子分散的活性位点与反应物之间的界面分子相互作用,以深入了解催化机制和动力学。氮掺杂碳纳米管(NCNT)、原子分散在NCNT上的锰(Mn-NCNT)和过一硫酸盐(PMS)被用作研究平台。发现原子分散的 Mn 位点上的 PMS 吸附在 NCNT 上执行内球相互作用,而不是外球相互作用。在有机污染物存在的情况下,内球相互作用导致有效的电子转移和强大的 PMS 吸附,因此类芬顿催化的净化效率很高。理论计算、X射线电子能谱、X射线吸收谱表明Mn-N部分与PMS之间形成了化学键,特别是离子键。相反,N掺杂表面通过范德华相互作用吸附PMS。这些发现提供了对固/液界面过程的深入理解,并为单原子催化剂的优越性提供了新的视角。
更新日期:2023-12-17
中文翻译:

理解Mn-N4位点多相芬顿催化中的界面分子相互作用和内层反应机制
本研究研究了类芬顿催化中原子分散的活性位点与反应物之间的界面分子相互作用,以深入了解催化机制和动力学。氮掺杂碳纳米管(NCNT)、原子分散在NCNT上的锰(Mn-NCNT)和过一硫酸盐(PMS)被用作研究平台。发现原子分散的 Mn 位点上的 PMS 吸附在 NCNT 上执行内球相互作用,而不是外球相互作用。在有机污染物存在的情况下,内球相互作用导致有效的电子转移和强大的 PMS 吸附,因此类芬顿催化的净化效率很高。理论计算、X射线电子能谱、X射线吸收谱表明Mn-N部分与PMS之间形成了化学键,特别是离子键。相反,N掺杂表面通过范德华相互作用吸附PMS。这些发现提供了对固/液界面过程的深入理解,并为单原子催化剂的优越性提供了新的视角。

































 京公网安备 11010802027423号
京公网安备 11010802027423号