当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface-enhanced bimetallic effect of Au-Pd by internal electromagnetic fields from Au@Cu2O for efficient electrochemical nitrate reduction to ammonia
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-12-17 , DOI: 10.1016/j.cej.2023.148152
Xiaojing Yu , Shengjun Du , Zhanzhi Xu , Jing He , Fuzhu Liu , Bin Wang , Shaodong Sun , Yufei Tang , Kang Zhao
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-12-17 , DOI: 10.1016/j.cej.2023.148152
Xiaojing Yu , Shengjun Du , Zhanzhi Xu , Jing He , Fuzhu Liu , Bin Wang , Shaodong Sun , Yufei Tang , Kang Zhao
|
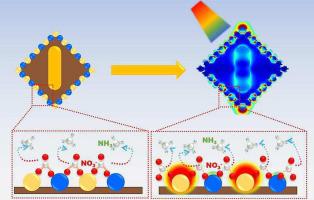
Electrochemical synthesis of ammonia from nitrate is an attractive approach to produce high-value “zero-carbon” fuel. However, this nitrate-to-ammonia technology is still restricted by its unsatisfactory ammonia conversion, which is resulted from the inevitable by-products formed during the complicated proton-coupled electron transfer process. In this study, Au NR@Cu2 O-AuPd NPs were designed by taking advantage of the internal electromagnetic field derived from Au NR for enhanced nitrate-to-ammonia performance. The constructed Au NR@Cu2 O-AuPd NPs exhibited a high yield of ammonia (4587.00 μg h−1 mgcat. −1 ) and FE(93.09 %) in neutral solution (pH = 7), which is attributed to the different adsorption capacities of the surface bimetals for nitrate and the reaction intermediates of nitrate reduction to NH3 . Under the irradiation of light, the surface bimetals showed different responsiveness to the generated internal electromagnetic field derived from Au NR, enabling a further increase in the yield of NH3 to 5328.99 μg h−1 mgcat. −1 . This work provides new insights into enhancing the conversion of nitrate to NH3 by electrocatalytic reduction through photo-modulation of the adsorption capacity on the catalyst surface.
中文翻译:

Au@Cu2O内部电磁场增强Au-Pd的表面增强双金属效应,有效电化学还原硝酸盐生成氨
从硝酸盐电化学合成氨是生产高价值“零碳”燃料的一种有吸引力的方法。然而,这种硝酸盐制氨技术仍然受到其氨转化率不理想的限制,这是由于复杂的质子耦合电子转移过程中不可避免地形成的副产物造成的。在这项研究中,Au NR@Cu2O-AuPd NPs 的设计利用了 Au NR 产生的内部电磁场来增强硝酸盐到氨的性能。构建的Au NR@Cu2O-AuPd NPs在中性溶液(pH = 7)中表现出高氨产率(4587.00 μg h−1mgcat.−1)和FE(93.09 %),这归因于Au NR@Cu2O-AuPd NPs的不同吸附能力硝酸盐的表面双金属以及硝酸盐还原为NH3的反应中间体。在光照射下,表面双金属对Au NR产生的内部电磁场表现出不同的响应性,使得NH3的产率进一步增加至5328.99 μg h−1mgcat.−1。这项工作为通过光调制催化剂表面吸附能力的电催化还原增强硝酸盐向NH3的转化提供了新的见解。
更新日期:2023-12-17
中文翻译:

Au@Cu2O内部电磁场增强Au-Pd的表面增强双金属效应,有效电化学还原硝酸盐生成氨
从硝酸盐电化学合成氨是生产高价值“零碳”燃料的一种有吸引力的方法。然而,这种硝酸盐制氨技术仍然受到其氨转化率不理想的限制,这是由于复杂的质子耦合电子转移过程中不可避免地形成的副产物造成的。在这项研究中,Au NR@Cu2O-AuPd NPs 的设计利用了 Au NR 产生的内部电磁场来增强硝酸盐到氨的性能。构建的Au NR@Cu2O-AuPd NPs在中性溶液(pH = 7)中表现出高氨产率(4587.00 μg h−1mgcat.−1)和FE(93.09 %),这归因于Au NR@Cu2O-AuPd NPs的不同吸附能力硝酸盐的表面双金属以及硝酸盐还原为NH3的反应中间体。在光照射下,表面双金属对Au NR产生的内部电磁场表现出不同的响应性,使得NH3的产率进一步增加至5328.99 μg h−1mgcat.−1。这项工作为通过光调制催化剂表面吸附能力的电催化还原增强硝酸盐向NH3的转化提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号