Phytomedicine ( IF 6.7 ) Pub Date : 2023-12-17 , DOI: 10.1016/j.phymed.2023.155295 Han Huang , Qi Gu , Si-Ming Nie , Jian-Dong Wang , Heng Zhao , Bo-Wen Zhai , Mao-Yu Zhang , Yu-Jie Fu
|
Background
Geniposidic acid (GPA) alleviates oxidative stress and inflammation in mice However, whether it can effectively regulate lipid accumulation and prevent hyperlipidemia requires further investigation.
Purpose
This study combined the untargeted metabolomics of cells and a Caenorhabditis elegans model to evaluate the anti-hyperlipidemic potential of GPA by modulating oxidative stress and regulating lipid metabolism. A golden hamster model of hyperlipidemia was used to further validate the lipid-lowering effect and mechanism of action of GPA.
Methods
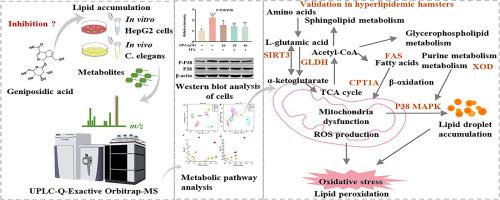
Chemical staining, immunofluorescence, and flow cytometry were performed to examine the effects of GPA on lipid accumulation and oxidative stress. Untargeted metabolomic analysis of cells and C. elegans was performed using ultra-performance liquid chromatography coupled with quadrupole electrostatic field Orbitrap high-resolution mass spectrometry (UPLC-Q-Orbitrap MS) to identify biomarkers altered by GPA action, analyze the affected metabolic pathways, and validate the mechanisms by which GPA regulates lipid metabolism and oxidative stress. A golden hamster model of hyperlipidemia was established to test the lipid-lowering effects of GPA. Body weight, biochemical markers, rate-limiting enzymes, and key proteins were assessed. Hematoxylin and eosin (H&E) and Oil Red O staining were performed.
Results
Phenotypic data showed that GPA decreased free fatty acid (FFA)-induced lipid buildup and high reactive oxygen species (ROS) levels, reversed the decrease in mitochondrial membrane potential (MMP), and increased the cellular reduced glutathione/oxidized glutathione disulfide (GSH/GSSG) ratio. GPA also reduces high glucose-induced lipid build-up and ROS production in C. elegans. Metabolomic analysis showed that GPA affected purine, lipid, and amino acid metabolism. Moreover, GPA inhibited xanthine oxidase (XOD), glutamate dehydrogenase (GLDH), fatty acid synthase (FAS), phosphorylation of P38 MAPK, and upregulated the expression of SIRT3 and CPT1A protein production to control lipid metabolism and produce antioxidant benefits in cells and golden hamsters.
Conclusion
Current evidence suggests that GPA can effectively regulate lipid metabolism and the oxidative stress response, and has the potential to prevent hyperlipidemia. This study also provided an effective method for evaluating the mechanism of action of GPA.
中文翻译:

非靶向代谢组学揭示京尼平苷酸对 HepG2 细胞和秀丽隐杆线虫脂质积累的调节作用,并在高脂仓鼠中进行验证
背景
京尼平苷酸(GPA)减轻小鼠氧化应激和炎症,但其是否能有效调节脂质堆积、预防高脂血症还需要进一步研究。
目的
本研究结合细胞非靶向代谢组学和秀丽隐杆线虫模型,通过调节氧化应激和调节脂质代谢来评估 GPA 的抗高血脂潜力。采用高脂血症金黄地鼠模型进一步验证GPA的降脂作用及作用机制。
方法
采用化学染色、免疫荧光和流式细胞术检查 GPA 对脂质积累和氧化应激的影响。使用超高效液相色谱结合四极静电场 Orbitrap 高分辨率质谱 (UPLC-Q-Orbitrap MS) 对细胞和线虫进行非靶向代谢组学分析,以识别 GPA 作用改变的生物标志物,分析受影响的代谢途径,并验证 GPA 调节脂质代谢和氧化应激的机制。建立高脂血症金黄地鼠模型,检测GPA的降脂作用。评估了体重、生化标志物、限速酶和关键蛋白质。进行苏木精和伊红 (H&E) 和油红 O 染色。
结果
表型数据显示,GPA 降低了游离脂肪酸(FFA) 诱导的脂质堆积和高活性氧(ROS) 水平,逆转了线粒体膜电位 (MMP) 的下降,并增加了细胞还原型谷胱甘肽/氧化型谷胱甘肽二硫化物 (GSH/ GSSG)比率。GPA 还可以减少线虫中高葡萄糖诱导的脂质堆积和 ROS 产生。代谢组学分析表明,GPA 影响嘌呤、脂质和氨基酸代谢。此外,GPA 抑制黄嘌呤氧化酶 (XOD)、谷氨酸脱氢酶(GLDH)、脂肪酸合酶(FAS)、P38 MAPK 的磷酸化,并上调 SIRT3 和 CPT1A 蛋白表达的表达,以控制脂质代谢并在细胞和黄金中产生抗氧化功效。仓鼠。
结论
目前的证据表明,GPA可以有效调节脂质代谢和氧化应激反应,并具有预防高脂血症的潜力。本研究也为评价GPA的作用机制提供了有效的方法。








































 京公网安备 11010802027423号
京公网安备 11010802027423号