目的
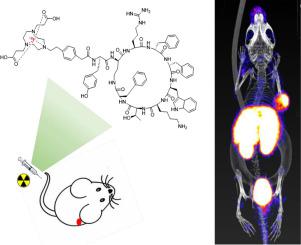
合成了一种新型18 F-放射性标记的生长抑素类似物 [Al 18 F]NODA-MPAA-HTA,并对其神经内分泌肿瘤 (NET) 的正电子发射断层扫描 (PET) 成像进行了评估。 [Al 18 F]NODA-MPAA-HTA 是通过将18 F 核素与修饰的 KE108 肽(一种对所有五种生长抑素受体亚型 (SSTR 1-5) 具有高亲和力的生长抑素类似物)通过偶联双功能螯合剂结合来设计和合成的(NODA)靶向生长抑素受体(SSTR)阳性肿瘤。
方法
将SSTRs靶向药效团KE108肽的氨基与NODA的羧基通过缩合反应缀合,得到标记前体[Al 18 F]NODA-MPAA-HTA,其前体通过Fmoc得到固相方法。使用螯合剂-生物分子缀合物的Al 18 F标记的新方法来合成[Al 18 F]NODA-MPAA-HTA。 [Al 18 F]NODA-MPAA-HTA 的体外稳定性通过在盐水或牛血清中孵育2小时来评价。进一步研究了 [Al 18 F]NODA-MPAA-HTA 的离体生物分布和体内成像,以评估其 SSTR 靶向能力以及使用 PET 成像诊断 NET 的可行性。
结果
[Al 18 F]NODA-MPAA-HTA 使用一步18 F-AlF 标记程序合成,具有中等的放射化学产率(60–80%,非衰变校正)和高放射化学纯度(>95%)。它表现出良好的亲水性和优异的体外稳定性,摩尔活性为122 GBq/μmol。在30分钟和60分钟时,HEK293-SSTR2细胞对[Al 18 F]NODA-MPAA-HTA的摄取分别为5.47±0.97%/105个细胞和12.11±0.32%/105个细胞。 [Al 18 F]NODA-MPAA-HTA 对SSTR2 的亲和力测定为8.77 ± 1.14 nM。在HEK293-SSTR2荷瘤小鼠的显微PET成像中,[Al 18 F]NODA-MPAA-HTA显示出高肿瘤放射性摄取和高肿瘤与肌肉比率。生物分布结果证实,肿瘤中的放射性摄取明显高于肌肉中的5倍以上(P<0 id=93>18 F]NODA-MPAA-HTA表明体内没有发生脱氟。这些初步结果结果为进一步研究Al ~(18) F标记的生长抑素类似物作为肿瘤探针用于NETs的PET成像提供了实验依据。
结论
Fluorine-18 广泛用作放射性核素,用于生产用于正电子发射断层扫描 (PET) 的放射性药物。由于其半衰期短(T1/2,109.8 分钟),其易于生产将有助于这种放射性药物的广泛传播。合成了高质量的[Al 18 F]NODA-MPAA-HTA,收率令人满意。这种放射性药物表现出更高的肿瘤摄取和更好的肿瘤与肌肉对比度,从而产生出色的图像质量。这些发现表明,新型18 F 标记的生长抑素类似物 [Al 18 F]NODA-MPAA-HTA 是一种很有前景的 NET PET 成像工具。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
18F-labeled somatostatin analogs for somatostatin receptors (SSTRs) targeted PET imaging of neuroendocrine tumors (NETs)
Purpose
A novel 18F-radiolabeled somatostatin analogue, [Al18F]NODA-MPAA-HTA, was synthesized and evaluated for positron emission tomography (PET) imaging of Neuroendocrine tumors (NETs). [Al18F]NODA-MPAA-HTA was designed and synthesized by conjugating 18F nuclide with a modified KE108 peptide, a somatostatin analog with high affinity for all five subtypes of somatostatin receptors (SSTR 1–5), through coupling a bifunctional chelator (NODA) to target somatostatin receptor (SSTR) positive tumors.
Methods
The amino group of KE108 peptide, a SSTRs-targeting pharmacophore, was conjugated with the carboxyl group of NODA by a condensation reaction to obtain the labeling precursor of [Al18F]NODA-MPAA-HTA, in which its precursor was obtained through Fmoc solid-phase methods. A novel methodology for Al18F labeling of chelating agent-biomolecule conjugates was used to synthesize [Al18F]NODA-MPAA-HTA. In vitro stabilities of [Al18F]NODA-MPAA-HTA were evaluated by incubating it in saline or bovine serum for 2 h. Ex vivo biodistribution and in vivo imaging of [Al18F]NODA-MPAA-HTA were further investigated to evaluate its SSTRs targeting ability and feasibility for the diagnosis of NETs using PET imaging.
Results
[Al18F]NODA-MPAA-HTA was synthesized using a one-step 18F-AlF labeling procedure resulting in moderate radiochemical yield (60–80 %, non-decay corrected) and high radiochemical purity (>95 %). It exhibited good hydrophilicity and excellent stability in vitro, with a molar activity of 122 GBq/μmol. At 30 min and 60 min, the uptake of [Al18F] NODA-MPAA-HTA by HEK293-SSTR2 cells was 5.47 ± 0.97 %/105 cells and 12.11 ± 0.32 %/105 cells, respectively. The affinity of [Al18F]NODA-MPAA-HTA for SSTR2 was determined to be 8.77 ± 1.14 nM. In micro-PET imaging of HEK293-SSTR2 tumor-bearing mice, [Al18F]NODA-MPAA-HTA showed high tumor uptake of radioactivity and a high tumor-to-muscle ratio. Biodistribution results confirmed that radioactivity uptake in the tumor was significantly higher than that in the muscle by more than five-fold (P<0.001). Furthermore, the relatively low bone uptake of [Al18F]NODA-MPAA-HTA suggested that defluorination did not occur in vivo. These preliminary results provide experimental evidence for further study of Al18F-labeled somatostatin analogues as tumor probes for PET imaging of NETs.
Conclusion
Fluorine-18 is widely used as a radionuclide for the production of radiopharmaceuticals for positron emission tomography (PET). Due to its short half-life (T1/2,109.8 min), its ease of production will facilitate the widespread dissemination of this radiopharmaceutical. A high-quality [Al18F]NODA-MPAA-HTA was synthesized with satisfactory yield. This radiopharmaceutical demonstrated higher tumor uptake and better tumor-to-muscle contrast, resulting to excellent image quality. These findings suggest that the novel 18F-labeled somatostatin analogue, [Al18F]NODA-MPAA-HTA, is a promising tool for PET imaging of NETs.
































 京公网安备 11010802027423号
京公网安备 11010802027423号