当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Novel PD-L1 Inhibitors That Induce the Dimerization, Internalization, and Degradation of PD-L1 Based on the Fragment Coupling Strategy
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-12-18 , DOI: 10.1021/acs.jmedchem.3c01534 Kaizhen Wang 1 , Xiangyu Zhang 1 , Yao Cheng 1 , Zhihao Qi 1 , Ke Ye 1 , Kuojun Zhang 1 , Sheng Jiang 1 , Yi Liu 1 , Yibei Xiao 1 , Tianyu Wang 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-12-18 , DOI: 10.1021/acs.jmedchem.3c01534 Kaizhen Wang 1 , Xiangyu Zhang 1 , Yao Cheng 1 , Zhihao Qi 1 , Ke Ye 1 , Kuojun Zhang 1 , Sheng Jiang 1 , Yi Liu 1 , Yibei Xiao 1 , Tianyu Wang 1
Affiliation
|
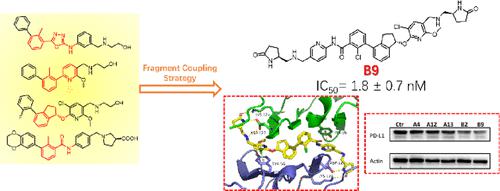
Tumor cells can evade immune surveillance through overexpressing programmed cell death-ligand 1 (PD-L1) to interact with programmed cell death-1 (PD-1). Besides, tumor-intrinsic PD-L1 is involved in tumor progression without interaction with PD-1, which provides more challenges for the discovery of PD-L1 inhibitors. Herein, we report the discovery of novel PD-L1 inhibitors using the fragment coupling strategy. Among them, B9 was found to inhibit the PD-1/PD-L1 interaction with the best IC50 value of 1.8 ± 0.7 nM. Beyond the blockade of the PD-1/PD-L1 axis, B9 promotes the dimerization, internalization, and degradation of PD-L1. Furthermore, B9 displayed high in vivo antitumor efficacy in the CT26 mouse model and activated the immune microenvironment and induced PD-L1 degradation of PD-L1 in the tumor. These results show that B9 is a promising lead PD-L1 inhibitor through the blockade of PD-1/PD-L1 interaction and functional inhibition of the PD-L1 signal pathway.
中文翻译:

基于片段偶联策略发现诱导 PD-L1 二聚化、内化和降解的新型 PD-L1 抑制剂
肿瘤细胞可以通过过度表达程序性细胞死亡配体1(PD-L1)与程序性细胞死亡-1(PD-1)相互作用来逃避免疫监视。此外,肿瘤固有的PD-L1在不与PD-1相互作用的情况下参与肿瘤进展,这为PD-L1抑制剂的发现提供了更多挑战。在此,我们报告了使用片段偶联策略发现的新型 PD-L1 抑制剂。其中, B9被发现可抑制PD-1/PD-L1相互作用,最佳IC 50值为1.8 ± 0.7 nM。除了阻断 PD-1/PD-L1 轴之外, B9还促进 PD-L1 的二聚化、内化和降解。此外, B9在CT26小鼠模型中表现出较高的体内抗肿瘤功效,并激活免疫微环境并诱导肿瘤中PD-L1的PD-L1降解。这些结果表明, B9通过阻断 PD-1/PD-L1 相互作用和功能性抑制 PD-L1 信号通路,成为一种有前景的先导 PD-L1 抑制剂。
更新日期:2023-12-18
中文翻译:

基于片段偶联策略发现诱导 PD-L1 二聚化、内化和降解的新型 PD-L1 抑制剂
肿瘤细胞可以通过过度表达程序性细胞死亡配体1(PD-L1)与程序性细胞死亡-1(PD-1)相互作用来逃避免疫监视。此外,肿瘤固有的PD-L1在不与PD-1相互作用的情况下参与肿瘤进展,这为PD-L1抑制剂的发现提供了更多挑战。在此,我们报告了使用片段偶联策略发现的新型 PD-L1 抑制剂。其中, B9被发现可抑制PD-1/PD-L1相互作用,最佳IC 50值为1.8 ± 0.7 nM。除了阻断 PD-1/PD-L1 轴之外, B9还促进 PD-L1 的二聚化、内化和降解。此外, B9在CT26小鼠模型中表现出较高的体内抗肿瘤功效,并激活免疫微环境并诱导肿瘤中PD-L1的PD-L1降解。这些结果表明, B9通过阻断 PD-1/PD-L1 相互作用和功能性抑制 PD-L1 信号通路,成为一种有前景的先导 PD-L1 抑制剂。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号