当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Selective Synthesis of Antitumor C5-Aminated Indoles via Neighboring Aldehyde Group Assisted Catellani Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2023-12-17 , DOI: 10.1021/acs.orglett.3c03932
Guangyuan Liu 1 , Mengzhu Zheng 1 , Rong Tian 2 , Yirong Zhou 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-12-17 , DOI: 10.1021/acs.orglett.3c03932
Guangyuan Liu 1 , Mengzhu Zheng 1 , Rong Tian 2 , Yirong Zhou 1
Affiliation
|
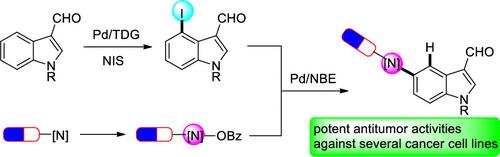
A palladium/norbornene (NBE) cooperative catalytic system was developed to access C5-aminated indoles, starting from readily available C4-idonated indoles. Good yields and exclusive site selectivity were achieved for a broad substrate scope, including drug molecule core architectures. Control experiments found that both aldehyde on the C3 position and sulfonyl protecting group on the N1 position were vital for the transformation. Preliminary bioactivity evaluation identified a promising leading compound 3af with potent antitumor proliferative activity against several cancer cells.
中文翻译:

通过邻近醛基辅助 Catellani 反应选点合成抗肿瘤 C5 胺化吲哚
开发了一种钯/降冰片烯 (NBE) 协同催化系统,从容易获得的 C4 胺化吲哚开始获取 C5 胺化吲哚。对于广泛的底物范围(包括药物分子核心结构),实现了良好的产率和独特的位点选择性。对照实验发现C3位上的醛和N1位上的磺酰基保护基对于转化至关重要。初步生物活性评估发现了一种有前景的先导化合物3af ,对多种癌细胞具有有效的抗肿瘤增殖活性。
更新日期:2023-12-17
中文翻译:

通过邻近醛基辅助 Catellani 反应选点合成抗肿瘤 C5 胺化吲哚
开发了一种钯/降冰片烯 (NBE) 协同催化系统,从容易获得的 C4 胺化吲哚开始获取 C5 胺化吲哚。对于广泛的底物范围(包括药物分子核心结构),实现了良好的产率和独特的位点选择性。对照实验发现C3位上的醛和N1位上的磺酰基保护基对于转化至关重要。初步生物活性评估发现了一种有前景的先导化合物3af ,对多种癌细胞具有有效的抗肿瘤增殖活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号