当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling the molecular mechanism of 1,3,2-dioxathiolane 2,2-dioxide in a propylene carbonate-based battery electrolyte
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-12-16 , DOI: 10.1016/j.molliq.2023.123817 Jaeho Lee , Kyoung-Hee Shin , Young-Kyu Han
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-12-16 , DOI: 10.1016/j.molliq.2023.123817 Jaeho Lee , Kyoung-Hee Shin , Young-Kyu Han
|
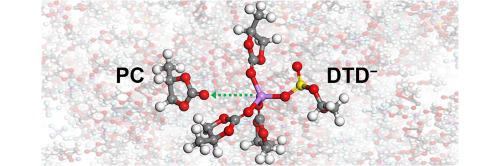
Propylene carbonate (PC)-based electrolytes are gaining attention as next-generation electrolytes for use in high-voltage and high-temperature environments due to their superior stability at high voltages and their wide operating temperature range. However, commercialization is challenged by the exfoliation of the graphite anode, which is caused by the co-intercalation of PC. Various additives have been devised to address this issue. 1,3,2-dioxathiolane 2,2-dioxide (DTD) exhibits outstanding capacity retention and lifespan characteristics in lithium-ion batteries in which PC-based electrolytes are used, but a molecular-level understanding of its operating mechanism remains elusive. According to our quantum static and dynamics calculations, the Li binding energy of DTD is much lower than that of PC, rendering its coordination ability insufficient to compete with PC. As a result, the neutral DTD does not play a role in favoring the desolvation of PC from the solvation structure. However, DTD is reduced prior to PC and shows a strong reduction tendency accompanied by ring-opening. Based on this, DTD in its anionic form participates in the Li solvation sheath through a solvent–additive exchange reaction to promote the desolvation of PC. We reveal that the use of the charges of the oxygen atoms bonded to Li ions to interpret the Li–solvent binding energies is inappropriate. Instead, we suggest the electrostatic potential minimum (ESP) as a useful and powerful descriptor. This work provides insights into the molecular characteristics and mechanisms of additives that enable PC-based electrolytes, offering guidance for the development of new additives.
中文翻译:

揭示碳酸丙烯酯电池电解液中 1,3,2-二氧硫杂环己烷 2,2-二氧化物的分子机制
碳酸丙烯酯(PC)基电解质由于其在高电压下具有优异的稳定性和较宽的工作温度范围,作为用于高电压和高温环境的下一代电解质而受到关注。然而,商业化面临着石墨阳极剥落的挑战,这是由 PC 共插层引起的。已设计出各种添加剂来解决这个问题。 1,3,2-二氧硫杂环己烷2,2-二氧化物 (DTD) 在使用 PC 电解质的锂离子电池中表现出出色的容量保持和寿命特性,但对其工作机制的分子水平理解仍然难以捉摸。根据我们的量子静态和动力学计算,DTD的Li结合能远低于PC,使其配位能力不足以与PC竞争。因此,中性 DTD 不能起到有利于 PC 从溶剂化结构中去溶剂化的作用。然而,DTD先于PC被还原,并显示出伴随开环的强烈还原趋势。基于此,阴离子形式的DTD通过溶剂-添加剂交换反应参与Li溶剂化鞘,促进PC的去溶剂化。我们发现,使用与锂离子键合的氧原子的电荷来解释锂-溶剂结合能是不合适的。相反,我们建议将静电势最小值 (ESP) 作为有用且强大的描述符。这项工作深入了解了用于 PC 电解质的添加剂的分子特征和机制,为新添加剂的开发提供了指导。
更新日期:2023-12-16
中文翻译:

揭示碳酸丙烯酯电池电解液中 1,3,2-二氧硫杂环己烷 2,2-二氧化物的分子机制
碳酸丙烯酯(PC)基电解质由于其在高电压下具有优异的稳定性和较宽的工作温度范围,作为用于高电压和高温环境的下一代电解质而受到关注。然而,商业化面临着石墨阳极剥落的挑战,这是由 PC 共插层引起的。已设计出各种添加剂来解决这个问题。 1,3,2-二氧硫杂环己烷2,2-二氧化物 (DTD) 在使用 PC 电解质的锂离子电池中表现出出色的容量保持和寿命特性,但对其工作机制的分子水平理解仍然难以捉摸。根据我们的量子静态和动力学计算,DTD的Li结合能远低于PC,使其配位能力不足以与PC竞争。因此,中性 DTD 不能起到有利于 PC 从溶剂化结构中去溶剂化的作用。然而,DTD先于PC被还原,并显示出伴随开环的强烈还原趋势。基于此,阴离子形式的DTD通过溶剂-添加剂交换反应参与Li溶剂化鞘,促进PC的去溶剂化。我们发现,使用与锂离子键合的氧原子的电荷来解释锂-溶剂结合能是不合适的。相反,我们建议将静电势最小值 (ESP) 作为有用且强大的描述符。这项工作深入了解了用于 PC 电解质的添加剂的分子特征和机制,为新添加剂的开发提供了指导。






























 京公网安备 11010802027423号
京公网安备 11010802027423号