当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced inhibition of human and rat aromatase activity by benzene ring substitutions in bisphenol A: QSAR structure-activity relationship and in silico docking analysis
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-12-14 , DOI: 10.1016/j.jhazmat.2023.133252
Jingyi Zheng 1 , Sailing Chen 1 , Han Lu 1 , Miaomiao Xia 1 , Shaowei Wang 1 , Xiaoheng Li 1 , Huitao Li 1 , Yiyan Wang 1 , Ren-Shan Ge 1 , Yi Liu 2
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-12-14 , DOI: 10.1016/j.jhazmat.2023.133252
Jingyi Zheng 1 , Sailing Chen 1 , Han Lu 1 , Miaomiao Xia 1 , Shaowei Wang 1 , Xiaoheng Li 1 , Huitao Li 1 , Yiyan Wang 1 , Ren-Shan Ge 1 , Yi Liu 2
Affiliation
|
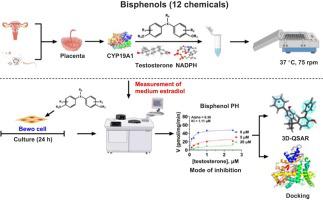
Bisphenol A (BPA) is a widely used plastic material, but its potential endocrine disrupting effect has restricted its use. The BPA alternatives have raised concerns. This study aimed to compare inhibitory potencies of 11 BPA analogues on human and rat placental aromatase (CYP19A1). The inhibitory potency on human CYP19A1 ranged from bisphenol H (IC50 , 0.93 μM) to tetramethyl BPA and tetrabromobisphenol S (ineffective at 100 μM) when compared to BPA (IC50 , 73.48 μM). Most of them were mixed/competitive inhibitors and inhibited estradiol production in human BeWo cells. Molecular docking analysis showed all BPA analogues bind to steroid active site or in between steroid and heme of CYP19A1 and form a hydrogen bond with catalytic residue Met374. Pharmacophore analysis showed that there were 4 hydrophobic regions for BPA analogues, with bisphenol H occupying 4 regions. Bivariate correlation analysis showed that LogP (lipophilicity) and LogS (water solubility) of BPA analogues were correlated with their IC50 values. Computerized drug metabolism and pharmacokinetics analysis showed that bisphenol H, tetrabromobisphenol A, and tetrachlorobisphenol A had low solubility, which might explain their weaker inhibition on estradiol production on BeWo cells. In conclusion, BPA analogues mostly can inhibit CYP19A1 and the lipophilicity determines their inhibitory strength.
中文翻译:

双酚 A 中苯环取代增强对人和大鼠芳香化酶活性的抑制:QSAR 构效关系和计算机对接分析
双酚 A (BPA) 是一种广泛使用的塑料材料,但其潜在的内分泌干扰作用限制了其使用。BPA 替代品引起了人们的担忧。本研究旨在比较 11 种 BPA 类似物对人和大鼠胎盘芳香化酶 (CYP19A1) 的抑制效力。与 BPA (IC50, 73.48 μM) 相比,对人CYP19A1的抑制效力范围为双酚 H (IC50, 0.93 μM) 到四甲基 BPA 和四溴双酚 S (在 100 μM 时无效)。它们中的大多数是混合/竞争性抑制剂,抑制人 BeWo 细胞中雌二醇的产生。分子对接分析显示,所有 BPA 类似物都与类固醇活性位点或类固醇CYP19A1血红素之间结合,并与催化残基 Met374 形成氢键。药效团分析表明,BPA 类似物有 4 个疏水区,双酚 H 占据 4 个区。双变量相关分析显示,BPA 类似物的 LogP (亲脂性) 和 LogS (水溶性) 与其 IC50 值相关。计算机化药物代谢和药代动力学分析显示,双酚 H、四溴双酚 A 和四氯双酚 A 的溶解度较低,这可能解释了它们对 BeWo 细胞上雌二醇产生的抑制较弱的原因。综上所述,BPA 类似物大多能抑制CYP19A1,亲脂性决定了它们的抑制强度。
更新日期:2023-12-14
中文翻译:

双酚 A 中苯环取代增强对人和大鼠芳香化酶活性的抑制:QSAR 构效关系和计算机对接分析
双酚 A (BPA) 是一种广泛使用的塑料材料,但其潜在的内分泌干扰作用限制了其使用。BPA 替代品引起了人们的担忧。本研究旨在比较 11 种 BPA 类似物对人和大鼠胎盘芳香化酶 (CYP19A1) 的抑制效力。与 BPA (IC50, 73.48 μM) 相比,对人CYP19A1的抑制效力范围为双酚 H (IC50, 0.93 μM) 到四甲基 BPA 和四溴双酚 S (在 100 μM 时无效)。它们中的大多数是混合/竞争性抑制剂,抑制人 BeWo 细胞中雌二醇的产生。分子对接分析显示,所有 BPA 类似物都与类固醇活性位点或类固醇CYP19A1血红素之间结合,并与催化残基 Met374 形成氢键。药效团分析表明,BPA 类似物有 4 个疏水区,双酚 H 占据 4 个区。双变量相关分析显示,BPA 类似物的 LogP (亲脂性) 和 LogS (水溶性) 与其 IC50 值相关。计算机化药物代谢和药代动力学分析显示,双酚 H、四溴双酚 A 和四氯双酚 A 的溶解度较低,这可能解释了它们对 BeWo 细胞上雌二醇产生的抑制较弱的原因。综上所述,BPA 类似物大多能抑制CYP19A1,亲脂性决定了它们的抑制强度。































 京公网安备 11010802027423号
京公网安备 11010802027423号