Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Restoring the Function of Thalamocortical Circuit Through Correcting Thalamic Kv3.2 Channelopathy Normalizes Fear Extinction Impairments in a PTSD Mouse Model
Advanced Science ( IF 14.3 ) Pub Date : 2023-12-16 , DOI: 10.1002/advs.202305939
Haoxiang Xiao 1 , Kaiwen Xi 1 , Kaifang Wang 2 , Yongsheng Zhou 1, 3 , Baowen Dong 4 , Jinyi Xie 1 , Yuqiao Xie 1 , Haifeng Zhang 1 , Guaiguai Ma 1 , Wenting Wang 1 , Dayun Feng 4 , Baolin Guo 1 , Shengxi Wu 1
Advanced Science ( IF 14.3 ) Pub Date : 2023-12-16 , DOI: 10.1002/advs.202305939
Haoxiang Xiao 1 , Kaiwen Xi 1 , Kaifang Wang 2 , Yongsheng Zhou 1, 3 , Baowen Dong 4 , Jinyi Xie 1 , Yuqiao Xie 1 , Haifeng Zhang 1 , Guaiguai Ma 1 , Wenting Wang 1 , Dayun Feng 4 , Baolin Guo 1 , Shengxi Wu 1
Affiliation
|
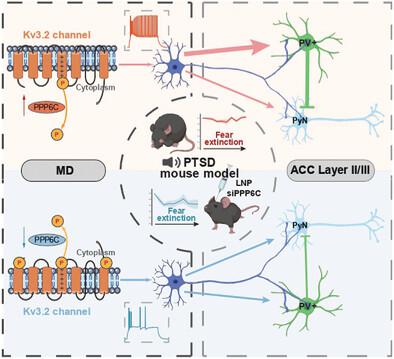
Impaired extinction of fear memory is one of the most common symptoms in post-traumatic stress disorder (PTSD), with limited therapeutic strategies due to the poor understanding of its underlying neural substrates. In this study, functional screening is performed and identified hyperactivity in the mediodorsal thalamic nucleus (MD) during fear extinction. Furthermore, the encoding patterns of the hyperactivated MD is investigated during persistent fear responses using multiple machine learning algorithms. The anterior cingulate cortex (ACC) is also identified as a functional downstream region of the MD that mediates the extinction of fear memory. The thalamocortical circuit is comprehensively analyzed and found that the MD-ACC parvalbumin interneurons circuit is preferentially enhanced in PTSD mice, disrupting the local excitatory and inhibitory balance. It is found that decreased phosphorylation of the Kv3.2 channel contributed to the hyperactivated MD, primarily to the malfunctioning thalamocortical circuit. Using a lipid nanoparticle-based RNA therapy strategy, channelopathy is corrected via a methoxylated siRNA targeting the protein phosphatase 6 catalytic subunit and restored fear memory extinction in PTSD mice. These findings highlight the function of the thalamocortical circuit in PTSD-related impaired extinction of fear memory and provide therapeutic insights into Kv3.2-targeted RNA therapy for PTSD.
中文翻译:

通过纠正丘脑 Kv3.2 通道病变恢复丘脑皮质回路的功能使 PTSD 小鼠模型中的恐惧消退障碍正常化
恐惧记忆消退受损是创伤后应激障碍(PTSD)最常见的症状之一,由于对其潜在神经基质了解甚少,治疗策略有限。在这项研究中,进行了功能筛查并确定了恐惧消退期间丘脑内侧核(MD)的过度活跃。此外,使用多种机器学习算法研究了持续恐惧反应期间过度激活的 MD 的编码模式。前扣带皮层 (ACC) 也被认为是介导恐惧记忆消退的 MD 下游功能区域。全面分析丘脑皮质回路发现,PTSD小鼠中MD-ACC小清蛋白中间神经元回路优先增强,破坏局部兴奋性和抑制性平衡。研究发现,Kv3.2 通道磷酸化的减少导致 MD 过度激活,主要是丘脑皮质回路发生故障。使用基于脂质纳米颗粒的 RNA 治疗策略,通过针对蛋白磷酸酶 6 催化亚基的甲氧基化 siRNA 纠正通道病变,并恢复 PTSD 小鼠的恐惧记忆消退。这些发现强调了丘脑皮质回路在 PTSD 相关的恐惧记忆消退受损中的功能,并为 Kv3.2 靶向 RNA 疗法治疗 PTSD 提供了治疗见解。
更新日期:2023-12-16
中文翻译:

通过纠正丘脑 Kv3.2 通道病变恢复丘脑皮质回路的功能使 PTSD 小鼠模型中的恐惧消退障碍正常化
恐惧记忆消退受损是创伤后应激障碍(PTSD)最常见的症状之一,由于对其潜在神经基质了解甚少,治疗策略有限。在这项研究中,进行了功能筛查并确定了恐惧消退期间丘脑内侧核(MD)的过度活跃。此外,使用多种机器学习算法研究了持续恐惧反应期间过度激活的 MD 的编码模式。前扣带皮层 (ACC) 也被认为是介导恐惧记忆消退的 MD 下游功能区域。全面分析丘脑皮质回路发现,PTSD小鼠中MD-ACC小清蛋白中间神经元回路优先增强,破坏局部兴奋性和抑制性平衡。研究发现,Kv3.2 通道磷酸化的减少导致 MD 过度激活,主要是丘脑皮质回路发生故障。使用基于脂质纳米颗粒的 RNA 治疗策略,通过针对蛋白磷酸酶 6 催化亚基的甲氧基化 siRNA 纠正通道病变,并恢复 PTSD 小鼠的恐惧记忆消退。这些发现强调了丘脑皮质回路在 PTSD 相关的恐惧记忆消退受损中的功能,并为 Kv3.2 靶向 RNA 疗法治疗 PTSD 提供了治疗见解。







































 京公网安备 11010802027423号
京公网安备 11010802027423号