当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Formal γ-C–H Functionalization of Carboxylic Acids Guided by Metal-Nitrenoids as an Unprecedented Mechanistic Motif
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-15 , DOI: 10.1021/jacs.3c11628 Sourav Pradhan 1, 2 , Jeonguk Kweon 1, 2 , Manoj Kumar Sahoo 1, 2 , Hoimin Jung 1, 2 , Joon Heo 1, 2 , Yeong Bum Kim 1, 2 , Dongwook Kim 1, 2 , Jung-Woo Park 1, 2 , Sukbok Chang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-15 , DOI: 10.1021/jacs.3c11628 Sourav Pradhan 1, 2 , Jeonguk Kweon 1, 2 , Manoj Kumar Sahoo 1, 2 , Hoimin Jung 1, 2 , Joon Heo 1, 2 , Yeong Bum Kim 1, 2 , Dongwook Kim 1, 2 , Jung-Woo Park 1, 2 , Sukbok Chang 1, 2
Affiliation
|
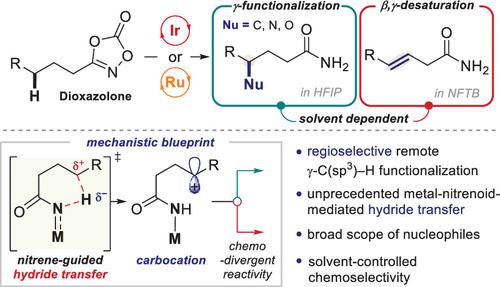
Harnessing the key intermediates in metal-catalyzed reactions is one of the most essential strategies in the development of selective organic transformations. The nitrogen group transfer reactivity of metal-nitrenoids to ubiquitous C–H bonds allows for diverse C–N bond formation to furnish synthetically valuable aminated products. In this study, we present an unprecedented reactivity of iridium and ruthenium nitrenoids to generate remote carbocation intermediates, which subsequently undergo nucleophile incorporation, thus developing a formal γ-C–H functionalization of carboxylic acids. Mechanistic investigations elucidated a unique singlet metal-nitrenoid reactivity to initiate an abstraction of γ-hydride to form the carbocation intermediate that eventually reacts with a broad range of carbon, nitrogen, and oxygen nucleophiles, as well as biorelevant molecules. Alternatively, the same intermediate can lead to deprotonation to afford β,γ-unsaturated amides in a less nucleophilic solvent.
中文翻译:

由金属氮烯类化合物引导的羧酸的形式 γ-C-H 官能化作为前所未有的机制基序
利用金属催化反应中的关键中间体是选择性有机转化发展中最重要的策略之一。金属氮素类化合物的氮基团转移反应性到普遍存在的 C-H 键,允许形成多种 C-N 键,从而提供具有合成价值的胺化产物。在这项研究中,我们提出了铱和钌氮烯类化合物前所未有的反应性,以产生远程碳阳离子中间体,随后进行亲核试剂掺入,从而形成了羧酸的正式γ-C-H官能化。机理研究阐明了独特的单线态金属氮烯类反应性,可引发γ-氢化物的提取,形成碳正离子中间体,最终与各种碳、氮和氧亲核试剂以及生物相关分子发生反应。或者,相同的中间体可以导致去质子化,以在亲核性较低的溶剂中提供 β,γ-不饱和酰胺。
更新日期:2023-12-15
中文翻译:

由金属氮烯类化合物引导的羧酸的形式 γ-C-H 官能化作为前所未有的机制基序
利用金属催化反应中的关键中间体是选择性有机转化发展中最重要的策略之一。金属氮素类化合物的氮基团转移反应性到普遍存在的 C-H 键,允许形成多种 C-N 键,从而提供具有合成价值的胺化产物。在这项研究中,我们提出了铱和钌氮烯类化合物前所未有的反应性,以产生远程碳阳离子中间体,随后进行亲核试剂掺入,从而形成了羧酸的正式γ-C-H官能化。机理研究阐明了独特的单线态金属氮烯类反应性,可引发γ-氢化物的提取,形成碳正离子中间体,最终与各种碳、氮和氧亲核试剂以及生物相关分子发生反应。或者,相同的中间体可以导致去质子化,以在亲核性较低的溶剂中提供 β,γ-不饱和酰胺。































 京公网安备 11010802027423号
京公网安备 11010802027423号