当前位置:
X-MOL 学术
›
J. Environ. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of 1-phenyl-3-(4-(pyridin-4-ylmethyl)phenyl)urea derivatives as robust corrosion inhibitors for mild steel in 1 M HCl environment: Insight from ,molecular, experimental, and microscopic-scale modelling approaches
Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2023-12-09 , DOI: 10.1016/j.jece.2023.111648 Arumugam Ramachandran , Panneerselvam Anitha , Sadhasivam Gnanavel , Subramania Angaiah
Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2023-12-09 , DOI: 10.1016/j.jece.2023.111648 Arumugam Ramachandran , Panneerselvam Anitha , Sadhasivam Gnanavel , Subramania Angaiah
|
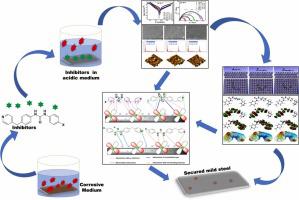
Cost-effective, novel urea derivatives, 1-phenyl-3-(4-(pyridin-4-ylmethyl)phenyl)urea (U-1), 1‐(4‐fluorophenyl)‐3‐(4‐(pyridin‐4‐ylmethyl)phenyl)urea (U-2), and 1‐(4‐methylphenyl)‐3‐(4‐(pyridin‐4‐ylmethyl)phenyl)urea (U-3) were synthesized to investigate as the corrosion inhibitors for mild steel/1 M HCl interface. Inhibition efficiency increases with the increasing inhibitor’s concentration but decreases with increasing temperature. Thermodynamic adsorption and activation parameters demonstrate that the adsorption of inhibitors follows Langmuir isotherm via physicochemical interactions. Potentiodynamic polarization measurements reveal that the inhibitors are mixed-type, impeding the charge-transfer process. Surface analysis supports a thin inhibitor film formation on the mild steel. The maximum inhibition efficiency of U-1, U-2 and U-3 are 94.97 ± 0.38%, 93.60 ± 0.62% and 97.42 ± 0.48% respectively. The calculated electrochemical impedance spectra (EIS) parameters such as ohmic resistance, double layer capacitance, relaxation time, effective thickness, and conductivity of grain and grain boundary are in-line with the efficiencies of NHUs. The performance of corrosion inhibitors follows a sequence U-3 > U-1 > U-2, emphasizing the influence of the substituent’s inductive effect (-CH > -H > -F) in inhibitors. Density functional theory (DFT) was performed to understand the correlation between molecular structure and inhibition efficiency. The Fukui indices confirm the presence of local attacking sites on the metal surface where inhibitors attack. A molecular dynamic (MD) simulation is used to determine the interaction energies between the inhibitors and mild steel. Radial Distribution Function (RDF) measurements confirm the existence of the chemical nature of the interaction. Furthermore, the diffusion coefficient of corrosive particles, self-diffusion coefficient of inhibitors, fractional free volume (FFV), and interaction between inhibitor’s film and corrosive particles are examined. Theoretical studies support experimental results.
中文翻译:

开发 1-苯基-3-(4-(吡啶-4-基甲基)苯基)脲衍生物作为 1 M HCl 环境中低碳钢的强效腐蚀抑制剂:来自分子、实验和微观尺度建模方法的见解
经济有效的新型脲衍生物,1-苯基-3-(4-(吡啶-4-基甲基)苯基)脲(U-1),1-(4-氟苯基)-3-(4-(吡啶-4)合成了1-(4-甲基苯基)-3-(4-(吡啶-4-基甲基)苯基)脲(U-2)和1-(4-甲基苯基)-3-(4-(吡啶-4-基甲基)苯基)脲(U-3),以研究其作为缓蚀剂的作用。低碳钢/1 M HCl 接口。抑制效率随着抑制剂浓度的增加而增加,但随着温度的升高而降低。热力学吸附和活化参数表明抑制剂的吸附通过物理化学相互作用遵循朗缪尔等温线。动电位极化测量表明抑制剂是混合型的,阻碍了电荷转移过程。表面分析支持在低碳钢上形成薄的抑制剂膜。U-1、U-2和U-3的最大抑制效率分别为94.97±0.38%、93.60±0.62%和97.42±0.48%。计算得到的电化学阻抗谱 (EIS) 参数,如欧姆电阻、双层电容、弛豫时间、有效厚度以及晶粒和晶界的电导率,与 NHU 的效率一致。缓蚀剂的性能遵循U-3>U-1>U-2的顺序,强调了取代基对缓蚀剂的诱导作用(-CH>-H>-F)的影响。采用密度泛函理论(DFT)来了解分子结构和抑制效率之间的相关性。福井指数证实了金属表面存在抑制剂攻击的局部攻击位点。分子动力学 (MD) 模拟用于确定抑制剂和低碳钢之间的相互作用能。径向分布函数 (RDF) 测量证实了相互作用的化学性质的存在。此外,还考察了腐蚀颗粒的扩散系数、缓蚀剂的自扩散系数、自由体积分数(FFV)以及缓蚀剂膜与腐蚀颗粒之间的相互作用。理论研究支持实验结果。
更新日期:2023-12-09
中文翻译:

开发 1-苯基-3-(4-(吡啶-4-基甲基)苯基)脲衍生物作为 1 M HCl 环境中低碳钢的强效腐蚀抑制剂:来自分子、实验和微观尺度建模方法的见解
经济有效的新型脲衍生物,1-苯基-3-(4-(吡啶-4-基甲基)苯基)脲(U-1),1-(4-氟苯基)-3-(4-(吡啶-4)合成了1-(4-甲基苯基)-3-(4-(吡啶-4-基甲基)苯基)脲(U-2)和1-(4-甲基苯基)-3-(4-(吡啶-4-基甲基)苯基)脲(U-3),以研究其作为缓蚀剂的作用。低碳钢/1 M HCl 接口。抑制效率随着抑制剂浓度的增加而增加,但随着温度的升高而降低。热力学吸附和活化参数表明抑制剂的吸附通过物理化学相互作用遵循朗缪尔等温线。动电位极化测量表明抑制剂是混合型的,阻碍了电荷转移过程。表面分析支持在低碳钢上形成薄的抑制剂膜。U-1、U-2和U-3的最大抑制效率分别为94.97±0.38%、93.60±0.62%和97.42±0.48%。计算得到的电化学阻抗谱 (EIS) 参数,如欧姆电阻、双层电容、弛豫时间、有效厚度以及晶粒和晶界的电导率,与 NHU 的效率一致。缓蚀剂的性能遵循U-3>U-1>U-2的顺序,强调了取代基对缓蚀剂的诱导作用(-CH>-H>-F)的影响。采用密度泛函理论(DFT)来了解分子结构和抑制效率之间的相关性。福井指数证实了金属表面存在抑制剂攻击的局部攻击位点。分子动力学 (MD) 模拟用于确定抑制剂和低碳钢之间的相互作用能。径向分布函数 (RDF) 测量证实了相互作用的化学性质的存在。此外,还考察了腐蚀颗粒的扩散系数、缓蚀剂的自扩散系数、自由体积分数(FFV)以及缓蚀剂膜与腐蚀颗粒之间的相互作用。理论研究支持实验结果。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号