Molecular Cell ( IF 14.5 ) Pub Date : 2023-12-12 , DOI: 10.1016/j.molcel.2023.11.015
Sihao Zheng 1 , Xiangyong Que 2 , Shuxian Wang 1 , Qi Zhou 2 , Xiaoke Xing 1 , Liang Chen 1 , Chunyan Hou 3 , Junfeng Ma 3 , Ping An 4 , Yihan Peng 3 , Yi Yao 1 , Qibin Song 1 , Juanjuan Li 5 , Pingfeng Zhang 1 , Huadong Pei 3
|
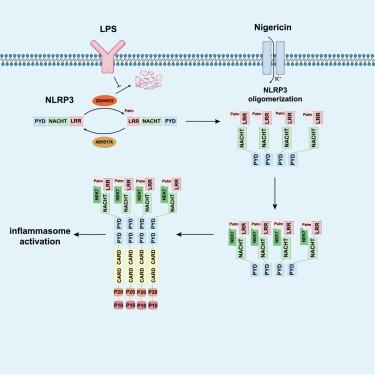
The nucleotide-binding domain (NBD), leucine-rich repeat (LRR), and pyrin domain (PYD)-containing protein 3 (NLRP3) inflammasome is a critical mediator of the innate immune response. How NLRP3 responds to stimuli and initiates the assembly of the NLRP3 inflammasome is not fully understood. Here, we found that a cellular metabolite, palmitate, facilitates NLRP3 activation by enhancing its S-palmitoylation, in synergy with lipopolysaccharide stimulation. NLRP3 is post-translationally palmitoylated by zinc-finger and aspartate-histidine-histidine-cysteine 5 (ZDHHC5) at the LRR domain, which promotes NLRP3 inflammasome assembly and activation. Silencing ZDHHC5 blocks NLRP3 oligomerization, NLRP3-NEK7 interaction, and formation of large intracellular ASC aggregates, leading to abrogation of caspase-1 activation, IL-1β/18 release, and GSDMD cleavage, both in human cells and in mice. ABHD17A depalmitoylates NLRP3, and one human-heritable disease-associated mutation in NLRP3 was found to be associated with defective ABHD17A binding and hyper-palmitoylation. Furthermore, Zdhhc5−/− mice showed defective NLRP3 inflammasome activation in vivo. Taken together, our data reveal an endogenous mechanism of inflammasome assembly and activation and suggest NLRP3 palmitoylation as a potential target for the treatment of NLRP3 inflammasome-driven diseases.
中文翻译:

ZDHHC5介导的NLRP3棕榈酰化促进NLRP3-NEK7相互作用和炎症小体激活
含有核苷酸结合结构域 (NBD)、富含亮氨酸重复序列 (LRR) 和吡啶结构域 (PYD) 的蛋白 3 (NLRP3) 炎性体是先天免疫反应的关键介质。 NLRP3 如何响应刺激并启动 NLRP3 炎性体的组装尚不完全清楚。在这里,我们发现细胞代谢物棕榈酸酯通过增强其 S-棕榈酰化与脂多糖刺激协同作用来促进 NLRP3 激活。 NLRP3 在 LRR 结构域被锌指和天冬氨酸-组氨酸-组氨酸-半胱氨酸 5 (ZDHHC5) 进行翻译后棕榈酰化,从而促进 NLRP3 炎性体组装和激活。沉默ZDHHC5可阻断 NLRP3 寡聚化、NLRP3-NEK7 相互作用以及大型细胞内 ASC 聚集体的形成,从而导致人类细胞和小鼠中 caspase-1 激活、IL-1β/18 释放和 GSDMD 裂解消失。 ABHD17A使 NLRP3 去棕榈酰化,并且发现 NLRP3 中的一种与人类遗传性疾病相关的突变与 ABHD17A 结合缺陷和过度棕榈酰化有关。此外, Zdhhc5 -/−小鼠在体内表现出有缺陷的 NLRP3 炎性体激活。综上所述,我们的数据揭示了炎症小体组装和激活的内源性机制,并表明 NLRP3 棕榈酰化作为治疗 NLRP3 炎症小体驱动疾病的潜在靶点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号