Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2023-12-15 , DOI: 10.1016/j.jelechem.2023.117991 Abdulah Javaid , Hassan Abdullah Khalid , Syed Ali Abbas Kazmi , Ghulam Ali
|
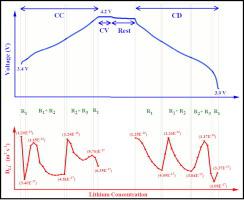
Cathode degradation during the charge and discharge process is inevitable due to phase transformations during the de-/lithiation process. Various factors such as temperature, cut-off voltage, and current rates tend to cause irreversibility in the phase transformations during cycling, resulting in the quick aging of lithium-ion batteries for electric vehicle applications. This study aims to evaluate the trends of the lithium-ion diffusion coefficient as a function of the lithium-ion concentration and potential in the lithium nickel cobalt aluminum oxide (NCA) cathode of the VTC5 lithium-ion cell through measurements by the galvanostatic intermittent titration technique (GITT). The lithium-ion cell has shown the lowest diffusion coefficient value of 1.08E−17 m2 s−1 at 0.2C rate at 25 °C temperature and the highest diffusion coefficient of 7.2829E−16 m2 s−1 at the lowest current rate of 0.05C rate and 45 °C temperature during discharging. The trend of diffusion coefficient values for lithium de-/intercalation with changing temperature and current rates is investigated and correlated with the phase transformations in the structure of the cathode and is indicative of a mixed-phase reaction mechanism. The electrochemical impedance spectroscopy data reinforces the dependence on lithium-ion diffusion on temperature, and voltage values and is supported by comparative analysis.
中文翻译:

镍钴铝酸锂基锂离子电池中锂扩散和过电势分析
由于脱/锂过程中的相变,充放电过程中的阴极退化是不可避免的。温度、截止电压和电流速率等各种因素往往会导致循环过程中相变的不可逆性,从而导致电动汽车应用的锂离子电池快速老化。本研究旨在通过恒电流间歇滴定法测量,评估 VTC5 锂离子电池镍钴铝酸锂 (NCA) 阴极中锂离子扩散系数随锂离子浓度和电位的变化趋势技术(GITT)。锂离子电池在 25 °C 温度和 0.2C 倍率下显示出最低扩散系数值为 1.08E −17 m 2 s −1 放电过程中,在0.05C速率和45℃温度的最低电流倍率下,扩散系数为7.2829E −16 m 2 s −1 。研究了锂脱嵌/嵌入的扩散系数值随温度和电流速率变化的趋势,并将其与阴极结构中的相变相关联,并表明了混合相反应机制。电化学阻抗谱数据强化了锂离子扩散对温度和电压值的依赖性,并得到比较分析的支持。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号