当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial Water Molecules as Agents for Phase Change Control and Proton Conductivity Enhancement in the Ammonium Vanadyl Tartrate System
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-12-15 , DOI: 10.1021/acs.inorgchem.3c02605 Marko Dunatov 1 , Krešimir Molčanov 1 , Zoran Štefanić 1 , Robert Kruk 2 , Lidija Androš Dubraja 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-12-15 , DOI: 10.1021/acs.inorgchem.3c02605 Marko Dunatov 1 , Krešimir Molčanov 1 , Zoran Štefanić 1 , Robert Kruk 2 , Lidija Androš Dubraja 1
Affiliation
|
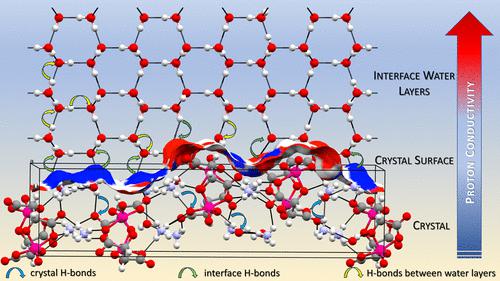
This study demonstrates the reversible structural transformation, single-crystal-to-single-crystal, of the ammonium vanadyl (L-tartrate) complex salt from the hydrate phase to the anhydrous phase. The transformation can be initiated by stimuli, such as temperature, humidity, or vacuum conditions. The hydrate and anhydrous phases exhibit a tetragonal structure (P41212), with marked differences in hydrogen bonding due to the presence or absence of one water molecule per asymmetric unit. The intricate relationship between crystal packing and intermolecular interactions in the hydrate phase was investigated by crystallographic charge density analysis revealing, at the molecular level, the reasons for the observed 5 orders of magnitude higher proton conductivity of the hydrate phase compared to that of the anhydrous phase. To gain further insight into the processes occurring at the surfaces of grain boundaries and the proton transfer mechanisms in this system, rehydration of the complex salt was carried out by using D2O instead of H2O and monitored by in situ ATR-FTIR spectroscopy. The results highlight the critical role of interfacial water molecules in driving structural transformations and influencing proton conductivity.
中文翻译:

界面水分子作为酒石酸氧钒体系中相变控制和质子电导率增强剂
本研究证明了氧钒酸铵( L-酒石酸)复合盐从水合物相到无水相的可逆结构转变,即单晶到单晶。这种转变可以由温度、湿度或真空条件等刺激引发。水合物和无水相呈现四方结构( P 4 1 2 1 2),由于每个不对称单元是否存在一个水分子,氢键存在显着差异。通过晶体电荷密度分析研究了水合物相中晶体堆积和分子间相互作用之间的复杂关系,揭示了在分子水平上观察到的水合物相质子电导率比无水相高 5 个数量级的原因。为了进一步了解晶界表面发生的过程以及该系统中的质子转移机制,使用 D 2 O 代替 H 2 O 进行复合盐的再水化,并通过原位ATR-FTIR 光谱进行监测。结果强调了界面水分子在驱动结构转变和影响质子电导率方面的关键作用。
更新日期:2023-12-15
中文翻译:

界面水分子作为酒石酸氧钒体系中相变控制和质子电导率增强剂
本研究证明了氧钒酸铵( L-酒石酸)复合盐从水合物相到无水相的可逆结构转变,即单晶到单晶。这种转变可以由温度、湿度或真空条件等刺激引发。水合物和无水相呈现四方结构( P 4 1 2 1 2),由于每个不对称单元是否存在一个水分子,氢键存在显着差异。通过晶体电荷密度分析研究了水合物相中晶体堆积和分子间相互作用之间的复杂关系,揭示了在分子水平上观察到的水合物相质子电导率比无水相高 5 个数量级的原因。为了进一步了解晶界表面发生的过程以及该系统中的质子转移机制,使用 D 2 O 代替 H 2 O 进行复合盐的再水化,并通过原位ATR-FTIR 光谱进行监测。结果强调了界面水分子在驱动结构转变和影响质子电导率方面的关键作用。





























 京公网安备 11010802027423号
京公网安备 11010802027423号