当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational design of an imine reductase: mechanism-guided stereoselectivity reversion and interface stabilization
Chemical Science ( IF 7.6 ) Pub Date : 2023-12-15 , DOI: 10.1039/d3sc04636b Kai Wu 1 , Jinrong Yan 2, 3 , Qinde Liu 1, 4 , Xiaojing Wang 1 , Piaoru Wu 1 , Yiyang Cao 1 , Xiuhong Lu 1 , Yixin Xu 1 , Junhai Huang 2, 3 , Lei Shao 1, 3
Chemical Science ( IF 7.6 ) Pub Date : 2023-12-15 , DOI: 10.1039/d3sc04636b Kai Wu 1 , Jinrong Yan 2, 3 , Qinde Liu 1, 4 , Xiaojing Wang 1 , Piaoru Wu 1 , Yiyang Cao 1 , Xiuhong Lu 1 , Yixin Xu 1 , Junhai Huang 2, 3 , Lei Shao 1, 3
Affiliation
|
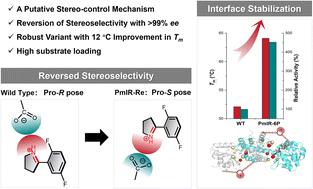
Imine reductases (IREDs) are important biocatalysts in the asymmetric synthesis of chiral amines. However, a detailed understanding of the stereocontrol mechanism of IRED remains incomplete, making the design of IRED for producing the desired amine enantiomers challenging. In this study, we investigated the stereoselective catalytic mechanism and designed an (R)-stereoselective IRED from Paenibacillus mucilaginosus (PmIR) using pharmaceutically relevant 2-aryl-substituted pyrrolines as substrates. A putative mechanism for controlling stereoselectivity was proposed based on the crucial role of electrostatic interactions in controlling iminium cation orientation and employed to achieve complete inversion of stereoselectivity in PmIR using computational design. The variant PmIR-Re (Q138M/P140M/Y187E/Q190A/D250M/R251N) exhibited opposite (S)-stereoselectivity, with >96% enantiomeric excess (ee) towards tested 2-aryl-substituted pyrrolines. Computational tools were employed to identify stabilizing mutations at the interface between the two subunits. The variant PmIR-6P (P140A/Q190S/R251N/Q217E/A257R/T277M) showed a nearly 5-fold increase in activity and a 12 °C increase in melting temperature. The PmIR-6P successfully produced (R)-2-(2,5-difluorophenyl)-pyrrolidine, a key chiral pharmaceutical intermediate, at a concentration of 400 mM with an ee exceeding 99%. This study provides insight into the stereocontrol elements of IREDs and demonstrates the potential of computational design for tailored stereoselectivity and thermal stability.
中文翻译:

亚胺还原酶的计算设计:机制引导的立体选择性反转和界面稳定
亚胺还原酶(IRED)是手性胺不对称合成中的重要生物催化剂。然而,对IRED立体控制机制的详细了解仍然不完整,这使得设计IRED来生产所需的胺对映体具有挑战性。在本研究中,我们研究了立体选择性催化机制,并使用药学相关的 2-芳基取代吡咯啉作为底物,从粘液类芽孢杆菌( Pm IR) 中设计了 ( R )-立体选择性 IRED。基于静电相互作用在控制亚胺阳离子取向中的关键作用,提出了一种控制立体选择性的假定机制,并利用计算设计实现了Pm IR 中立体选择性的完全反转。变体Pm IR-Re (Q138M/P140M/Y187E/Q190A/D250M/R251N) 表现出相反的 ( S )-立体选择性,对测试的 2-芳基取代的吡咯啉具有 >96% 对映体过量 (ee)。采用计算工具来识别两个亚基之间界面的稳定突变。变体Pm IR-6P (P140A/Q190S/R251N/Q217E/A257R/T277M) 的活性增加了近 5 倍,熔解温度增加了 12 °C。 Pm IR-6P成功生产了关键的手性药物中间体( R )-2-(2,5-二氟苯基)-吡咯烷,浓度为400 mM,ee超过99%。这项研究提供了对 IRED 立体控制元件的深入了解,并展示了计算设计在定制立体选择性和热稳定性方面的潜力。
更新日期:2023-12-15
中文翻译:

亚胺还原酶的计算设计:机制引导的立体选择性反转和界面稳定
亚胺还原酶(IRED)是手性胺不对称合成中的重要生物催化剂。然而,对IRED立体控制机制的详细了解仍然不完整,这使得设计IRED来生产所需的胺对映体具有挑战性。在本研究中,我们研究了立体选择性催化机制,并使用药学相关的 2-芳基取代吡咯啉作为底物,从粘液类芽孢杆菌( Pm IR) 中设计了 ( R )-立体选择性 IRED。基于静电相互作用在控制亚胺阳离子取向中的关键作用,提出了一种控制立体选择性的假定机制,并利用计算设计实现了Pm IR 中立体选择性的完全反转。变体Pm IR-Re (Q138M/P140M/Y187E/Q190A/D250M/R251N) 表现出相反的 ( S )-立体选择性,对测试的 2-芳基取代的吡咯啉具有 >96% 对映体过量 (ee)。采用计算工具来识别两个亚基之间界面的稳定突变。变体Pm IR-6P (P140A/Q190S/R251N/Q217E/A257R/T277M) 的活性增加了近 5 倍,熔解温度增加了 12 °C。 Pm IR-6P成功生产了关键的手性药物中间体( R )-2-(2,5-二氟苯基)-吡咯烷,浓度为400 mM,ee超过99%。这项研究提供了对 IRED 立体控制元件的深入了解,并展示了计算设计在定制立体选择性和热稳定性方面的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号