Phytomedicine ( IF 6.7 ) Pub Date : 2023-12-14 , DOI: 10.1016/j.phymed.2023.155263 Hongqing Chen 1 , Qiongying Hu 1 , Tian Wen 1 , Liuling Luo 2 , Lu Liu 2 , Lun Wang 3 , Xiaofei Shen 4
|
Background
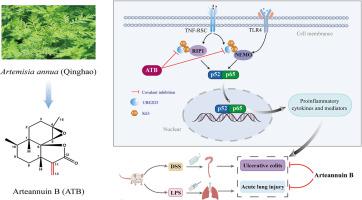
Anomalous activation of NF-κB signaling is associated with many inflammatory disorders, such as ulcerative colitis (UC) and acute lung injury (ALI). NF-κB activation requires the ubiquitination of receptor-interacting protein 1 (RIP1) and NF-κB essential modulator (NEMO). Therefore, inhibition of ubiquitation of RIP1 and NEMO may serve as a potential approach for inhibiting NF-κB activation and alleviating inflammatory disorders.
Purpose
Here, we identified arteannuin B (ATB), a sesquiterpene lactone found in the traditional Chinese medicine Artemisia annua that is used to treat malaria and inflammatory diseases, as a potent anti-inflammatory compound, and then characterized the putative mechanisms of its anti-inflammatory action.
Methods
Detections of inflammatory mediators and cytokines in LPS- or TNF-α-stimulated murine macrophages using RT-qPCR, ELISA, and western blotting, respectively. Western blotting, CETSA, DARTS, MST, gene knockdown, LC-MS/MS, and molecular docking were used to determine the potential target and molecular mechanism of ATB. The pharmacological effects of ATB were further evaluated in DSS-induced colitis and LPS-induced ALI in vivo.
Results
ATB effectively diminished the generation of NO and PGE2 by down-regulating iNOS and COX2 expression, and decreased the mRNA expression and release of IL-1β, IL-6, and TNF-α in LPS-exposed RAW264.7 macrophages. The anti-inflammatory effect of ATB was further demonstrated in LPS-treated BMDMs and TNF-α-activated RAW264.7 cells. We further found that ATB obviously inhibited NF-κB activation induced by LPS or TNF-α in vitro. Moreover, compared with ATB, dihydroarteannuin B (DATB) which lost the unsaturated double bond, completely failed to repress LPS-induced NO release and NF-κB activation in vitro. Furthermore, UBE2D3, a ubiquitin-conjugating enzyme, was identified as the functional target of ATB, but not DATB. UBE2D3 knockdown significantly abolished ATB-mediated inhibition on LPS-induced NO production. Mechanistically, ATB could covalently bind to the catalytic cysteine 85 of UBE2D3, thereby inhibiting the function of UBE2D3 and preventing ubiquitination of RIP1 and NEMO. In vivo, ATB treatment exhibited robust protective effects against DSS-induced UC and LPS-induced ALI.
Conclusion
Our findings first demonstrated that ATB exerted anti-inflammatory functions by repression of NF-κB pathway via covalently binding to UBE2D3, and raised the possibility that ATB could be effective in the treatment of inflammatory diseases and other diseases associated with abnormal NF-κB activation.
中文翻译:

青蒿素 B 是一种来自青蒿的倍半萜内酯,通过抑制泛素结合酶 UBE2D3 介导的 NF-κB 激活来减轻炎症反应
背景
NF-κB 信号传导的异常激活与许多炎症性疾病相关,例如溃疡性结肠炎(UC) 和急性肺损伤 (ALI)。 NF-κB 激活需要受体相互作用蛋白 1 (RIP1) 和 NF-κB 必需调节剂 (NEMO) 的泛素化。因此,抑制RIP1和NEMO的泛素化可能成为抑制NF-κB激活和减轻炎症性疾病的潜在方法。
目的
在这里,我们鉴定出青蒿素 B (ATB),一种在中药青蒿中发现的倍半萜内酯,用于治疗疟疾和炎症性疾病,是一种有效的抗炎化合物,然后表征了其抗炎的假定机制。行动。
方法
分别使用 RT-qPCR、ELISA 和蛋白质印迹检测 LPS 或 TNF-α 刺激的小鼠巨噬细胞中的炎症介质和细胞因子。采用Western blotting、CETSA、DARTS、MST、基因敲低、LC-MS/MS、分子对接等手段确定ATB的潜在靶点和分子机制。在体内DSS 诱导的结肠炎和 LPS 诱导的 ALI 中进一步评估了 ATB 的药理作用。
结果
ATB通过下调 iNOS 和 COX2 表达,有效减少 NO 和 PGE 2的产生,并减少 LPS 暴露的 RAW264.7 巨噬细胞中 IL-1β、IL-6 和 TNF-α 的 mRNA 表达和释放。 ATB 的抗炎作用在 LPS 处理的 BMDM 和 TNF-α 激活的 RAW264.7 细胞中得到进一步证明。我们进一步发现ATB在体外明显抑制LPS或TNF-α诱导的NF-κB活化。此外,与ATB相比,失去不饱和双键的二氢青蒿素B(DATB)在体外完全不能抑制LPS诱导的NO释放和NF-κB激活。此外,UBE2D3(一种泛素结合酶)被确定为 ATB 的功能靶标,但不是 DATB。 UBE2D3 敲除显着消除了 ATB 介导的对 LPS 诱导的 NO 产生的抑制。从机制上来说,ATB可以与UBE2D3的催化半胱氨酸85共价结合,从而抑制UBE2D3的功能并阻止RIP1和NEMO的泛素化。在体内,ATB 治疗对 DSS 诱导的 UC 和 LPS 诱导的 ALI 表现出强大的保护作用。
结论
我们的研究结果首先证明ATB通过与UBE2D3共价结合抑制NF-κB通路来发挥抗炎功能,并提出了ATB可以有效治疗炎症性疾病和与NF-κB异常激活相关的其他疾病的可能性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号