当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structurally diverse rearranged sesquiterpenoids, including a pair of rare tautomers, from the aerial parts of Daphne penicillata
Phytochemistry ( IF 3.2 ) Pub Date : 2023-12-14 , DOI: 10.1016/j.phytochem.2023.113950
Peng Zhao 1 , Ben-Song Xin 1 , Li Ye 1 , Zhen-Tao Ma 1 , Guo-Dong Yao 1 , Rui Shi 2 , Xia-Hong He 2 , Bin Lin 3 , Xiao-Xiao Huang 4 , Shao-Jiang Song 1
Phytochemistry ( IF 3.2 ) Pub Date : 2023-12-14 , DOI: 10.1016/j.phytochem.2023.113950
Peng Zhao 1 , Ben-Song Xin 1 , Li Ye 1 , Zhen-Tao Ma 1 , Guo-Dong Yao 1 , Rui Shi 2 , Xia-Hong He 2 , Bin Lin 3 , Xiao-Xiao Huang 4 , Shao-Jiang Song 1
Affiliation
|
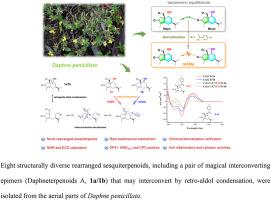
Eight structurally diverse rearranged sesquiterpenoids, including seven undescribed sesquiterpenoids ( and –) were obtained from the aerial parts of . , , and possess rare rearranged guaiane skeletons and represents the first example of rearranged carotene sesquiterpenoids. Their structures and absolute configurations were determined by extensive spectroscopic analyses, NMR and ECD calculations. Interestingly, and were a pair of magical interconverting epimers that may interconvert by retro-aldol condensation. The mechanism of interconversion has been demonstrated indirectly by 9-OH derivatization of and a hypothetical biogenetic pathway was proposed. All compounds were evaluated for anti-inflammatory and cytotoxic activities. Among them, and exhibited potential inhibitory activities on the production of NO against LPS-induced BV2 microglial cells.
中文翻译:

来自 Daphne penicillata 地上部分的结构多样的重排倍半萜类化合物,包括一对罕见的互变异构体
八种结构不同的重排倍半萜类化合物,包括七种未描述的倍半萜类化合物(和 -)是从 的地上部分获得的。 、 、 并拥有罕见的重排愈创木烷骨架,代表了重排胡萝卜素倍半萜类化合物的第一个例子。它们的结构和绝对构型是通过广泛的光谱分析、NMR 和 ECD 计算确定的。有趣的是, 和 是一对神奇的相互转换差向异构体,可以通过逆羟醛缩合相互转换。相互转化的机制已通过 9-OH 衍生化间接得到证实,并提出了一个假设的生物发生途径。评估所有化合物的抗炎和细胞毒活性。其中, 和 对LPS诱导的BV2小胶质细胞的NO产生表现出潜在的抑制活性。
更新日期:2023-12-14
中文翻译:

来自 Daphne penicillata 地上部分的结构多样的重排倍半萜类化合物,包括一对罕见的互变异构体
八种结构不同的重排倍半萜类化合物,包括七种未描述的倍半萜类化合物(和 -)是从 的地上部分获得的。 、 、 并拥有罕见的重排愈创木烷骨架,代表了重排胡萝卜素倍半萜类化合物的第一个例子。它们的结构和绝对构型是通过广泛的光谱分析、NMR 和 ECD 计算确定的。有趣的是, 和 是一对神奇的相互转换差向异构体,可以通过逆羟醛缩合相互转换。相互转化的机制已通过 9-OH 衍生化间接得到证实,并提出了一个假设的生物发生途径。评估所有化合物的抗炎和细胞毒活性。其中, 和 对LPS诱导的BV2小胶质细胞的NO产生表现出潜在的抑制活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号