当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptide Epimerase Responsible for d-Amino Acid Introduction in Poly-γ-glutamic Acid Biosynthesis
Biomacromolecules ( IF 5.5 ) Pub Date : 2023-12-14 , DOI: 10.1021/acs.biomac.3c01000 Hinata Kato 1 , Moeka Sakuta 1 , Takeshi Tsunoda 2 , Yu Nakashima 3 , Hiroyuki Morita 3 , Yasushi Ogasawara 2 , Tohru Dairi 2
Biomacromolecules ( IF 5.5 ) Pub Date : 2023-12-14 , DOI: 10.1021/acs.biomac.3c01000 Hinata Kato 1 , Moeka Sakuta 1 , Takeshi Tsunoda 2 , Yu Nakashima 3 , Hiroyuki Morita 3 , Yasushi Ogasawara 2 , Tohru Dairi 2
Affiliation
|
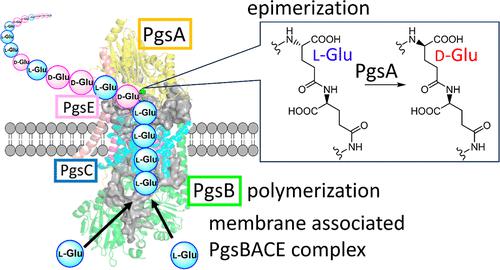
Poly-γ-glutamic acid (PGA) is a natural polymer of d- and/or l-glutamic acid (Glu) linked by isopeptide bonds. We recently showed that PGA synthetase, an enzyme complex composed of PgsB, PgsC, and PgsA, uses only l-Glu for polymerization, and d-Glu residues are introduced by peptide epimerization. However, it remains unclear which of the three enzymes is responsible for epimerization because in vitro functional characterization of the membrane-associated PgsBCA complex has never been successful. Here, we performed gene exchange experiments and showed that PgsA is responsible for the epimerization. Additionally, we identified a region in PgsA that modulates epimerization activity based on homology modeling from the recently solved structure of MslH, which showed 53% identity to PgsA. Our results suggested that d/l-ratios of the PGA product can be altered by introducing amino acid substitutions in this region, which will be useful for the production of PGA with controlled d/l-ratios.
中文翻译:

肽差向异构酶负责聚-γ-谷氨酸生物合成中 d-氨基酸的引入
聚-γ-谷氨酸(PGA)是通过异肽键连接的d-和/或l-谷氨酸(Glu)的天然聚合物。我们最近表明,PGA 合成酶是一种由 PgsB、PgsC 和 PgsA 组成的酶复合物,仅使用l -Glu 进行聚合,并通过肽差向异构化引入d -Glu 残基。然而,目前尚不清楚这三种酶中哪一种负责差向异构化,因为膜相关 PgsBCA 复合物的体外功能表征从未成功。在这里,我们进行了基因交换实验,结果表明 PgsA 负责差向异构化。此外,我们根据最近解析的 MslH 结构的同源模型,确定了 PgsA 中调节差向异构化活性的区域,该区域与 PgsA 有 53% 的同一性。我们的结果表明,可以通过在该区域引入氨基酸取代来改变 PGA 产品的d / l比,这将有助于生产具有受控d / l比的 PGA。
更新日期:2023-12-14
中文翻译:

肽差向异构酶负责聚-γ-谷氨酸生物合成中 d-氨基酸的引入
聚-γ-谷氨酸(PGA)是通过异肽键连接的d-和/或l-谷氨酸(Glu)的天然聚合物。我们最近表明,PGA 合成酶是一种由 PgsB、PgsC 和 PgsA 组成的酶复合物,仅使用l -Glu 进行聚合,并通过肽差向异构化引入d -Glu 残基。然而,目前尚不清楚这三种酶中哪一种负责差向异构化,因为膜相关 PgsBCA 复合物的体外功能表征从未成功。在这里,我们进行了基因交换实验,结果表明 PgsA 负责差向异构化。此外,我们根据最近解析的 MslH 结构的同源模型,确定了 PgsA 中调节差向异构化活性的区域,该区域与 PgsA 有 53% 的同一性。我们的结果表明,可以通过在该区域引入氨基酸取代来改变 PGA 产品的d / l比,这将有助于生产具有受控d / l比的 PGA。

































 京公网安备 11010802027423号
京公网安备 11010802027423号