当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tri-Stimulus-Responsive Hollow Mesoporous MnO2 Nanocarriers for Magnetic-Resonance-Imaging-Guided Synergistic Starvation/Photodynamic Therapy of Breast Cancer
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2023-12-14 , DOI: 10.1021/acsanm.3c05733 Rongjian Jiang 1, 2 , Lifeng Hang 2 , Wuming Li 2 , Huangsheng Ling 1, 2 , Haiying Wang 1, 2 , Qiang Lei 2 , Huanhuan Su 2 , Yiyu Chen 2 , Xiaofen Ma 2 , Guihua Jiang 1, 2
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2023-12-14 , DOI: 10.1021/acsanm.3c05733 Rongjian Jiang 1, 2 , Lifeng Hang 2 , Wuming Li 2 , Huangsheng Ling 1, 2 , Haiying Wang 1, 2 , Qiang Lei 2 , Huanhuan Su 2 , Yiyu Chen 2 , Xiaofen Ma 2 , Guihua Jiang 1, 2
Affiliation
|
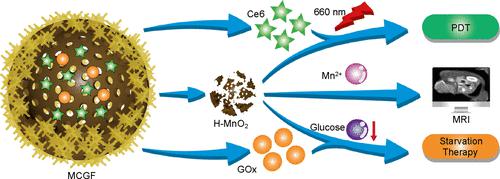
Hypoxia, a distinct feature of breast cancer, can affect the effectiveness of breast cancer therapy. To alleviate the hypoxic state of the tumor microenvironment and achieve synergistic starvation-photodynamic therapy (PDT), we have developed a simple and effective multifunctional nanoplatform. Spherical silica (SiO2) serves as a template for the synthesis of hollow mesoporous MnO2 (H-MnO2) nanoparticles, which act as the carriers of chlorin e6 (Ce6) and glucose oxidase (GOx). Subsequently, the particles are modified with the surfactant Pluronic F-127, leading to the formation of a H-MnO2/Ce6/GOx/F-127 (MCGF) nanoplatform. During tumor treatment, pH/glutathione/H2O2-responsive MCGF allows the controlled release of encapsulated Ce6 and GOx, avoiding pre-exposure to body fluids. Upon release, GOx oxidizes endogenous glucose to generate H2O2 and gluconic acid. The catalase-like H-MnO2 reacts with the endogenous and glucose-oxidation-generated H2O2 to produce O2. Reinforcement between these catalytic reactions promotes the O2-dependent Ce6-mediated PDT and GOx-induced starvation therapy. Moreover, T1-weighted magnetic resonance imaging (MRI) of Mn2+, which is produced by H-MnO2 degradation, allows for real-time tumor therapy monitoring. Both in vitro and in vivo experiments demonstrate that the MCGF nanoplatform allows MRI monitoring and achieves efficient starvation/PDT effects for synergistic breast cancer treatment. We believe that our findings will contribute to the development of nanotheranostic platforms for breast cancer starvation/PDT.
中文翻译:

三刺激响应空心介孔MnO2纳米载体用于磁共振成像引导的乳腺癌协同饥饿/光动力治疗
缺氧是乳腺癌的一个显着特征,会影响乳腺癌治疗的效果。为了缓解肿瘤微环境的缺氧状态并实现协同饥饿-光动力疗法(PDT),我们开发了一种简单有效的多功能纳米平台。以球形二氧化硅(SiO 2)为模板合成中空介孔MnO 2(H-MnO 2)纳米颗粒,作为二氢卟酚e6(Ce6)和葡萄糖氧化酶(GOx)的载体。随后,用表面活性剂 Pluronic F-127 对颗粒进行改性,形成 H-MnO 2 /Ce6/GOx/F-127 (MCGF) 纳米平台。在肿瘤治疗期间,pH/谷胱甘肽/H 2 O 2响应性MCGF允许封装的Ce6和GOx的受控释放,避免预先暴露于体液。释放后,GOx 氧化内源性葡萄糖,生成 H 2 O 2和葡萄糖酸。过氧化氢酶样H-MnO 2与内源性和葡萄糖氧化产生的H 2 O 2反应产生O 2。这些催化反应之间的强化促进了 O 2依赖性 Ce6 介导的 PDT 和 GOx 诱导的饥饿疗法。此外,H-MnO 2降解产生的Mn 2+的T1加权磁共振成像(MRI)允许实时肿瘤治疗监测。体外和体内实验均表明,MCGF 纳米平台可实现 MRI 监测,并实现有效的饥饿/PDT 效果,以实现乳腺癌的协同治疗。我们相信,我们的研究结果将有助于乳腺癌饥饿/PDT 纳米治疗平台的开发。
更新日期:2023-12-14
中文翻译:

三刺激响应空心介孔MnO2纳米载体用于磁共振成像引导的乳腺癌协同饥饿/光动力治疗
缺氧是乳腺癌的一个显着特征,会影响乳腺癌治疗的效果。为了缓解肿瘤微环境的缺氧状态并实现协同饥饿-光动力疗法(PDT),我们开发了一种简单有效的多功能纳米平台。以球形二氧化硅(SiO 2)为模板合成中空介孔MnO 2(H-MnO 2)纳米颗粒,作为二氢卟酚e6(Ce6)和葡萄糖氧化酶(GOx)的载体。随后,用表面活性剂 Pluronic F-127 对颗粒进行改性,形成 H-MnO 2 /Ce6/GOx/F-127 (MCGF) 纳米平台。在肿瘤治疗期间,pH/谷胱甘肽/H 2 O 2响应性MCGF允许封装的Ce6和GOx的受控释放,避免预先暴露于体液。释放后,GOx 氧化内源性葡萄糖,生成 H 2 O 2和葡萄糖酸。过氧化氢酶样H-MnO 2与内源性和葡萄糖氧化产生的H 2 O 2反应产生O 2。这些催化反应之间的强化促进了 O 2依赖性 Ce6 介导的 PDT 和 GOx 诱导的饥饿疗法。此外,H-MnO 2降解产生的Mn 2+的T1加权磁共振成像(MRI)允许实时肿瘤治疗监测。体外和体内实验均表明,MCGF 纳米平台可实现 MRI 监测,并实现有效的饥饿/PDT 效果,以实现乳腺癌的协同治疗。我们相信,我们的研究结果将有助于乳腺癌饥饿/PDT 纳米治疗平台的开发。





























 京公网安备 11010802027423号
京公网安备 11010802027423号