当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photosensitizer-singlet oxygen sensor conjugated silica nanoparticles for photodynamic therapy and bioimaging
Chemical Science ( IF 7.6 ) Pub Date : 2023-12-14 , DOI: 10.1039/d3sc03877g Jeladhara Sobhanan 1, 2 , Kenji Ono 3 , Takuya Okamoto 1, 4 , Makoto Sawada 3 , Paul S Weiss 5 , Vasudevanpillai Biju 1, 4
Chemical Science ( IF 7.6 ) Pub Date : 2023-12-14 , DOI: 10.1039/d3sc03877g Jeladhara Sobhanan 1, 2 , Kenji Ono 3 , Takuya Okamoto 1, 4 , Makoto Sawada 3 , Paul S Weiss 5 , Vasudevanpillai Biju 1, 4
Affiliation
|
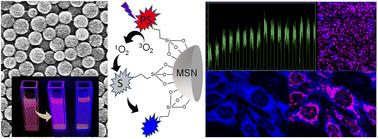
Intracellular singlet oxygen (1O2) generation and detection help optimize the outcome of photodynamic therapy (PDT). Theranostics programmed for on-demand phototriggered 1O2 release and bioimaging have great potential to transform PDT. We demonstrate an ultrasensitive fluorescence turn-on sensor-sensitizer-RGD peptide-silica nanoarchitecture and its 1O2 generation–releasing–storing–sensing properties at the single-particle level or in living cells. The sensor and sensitizer in the nanoarchitecture are an aminomethyl anthracene (AMA)-coumarin dyad and a porphyrin or CdSe/ZnS quantum dots (QDs), respectively. The AMA in the dyad quantitatively quenches the fluorescence of coumarin by intramolecular electron transfer, the porphyrin or QD moiety generates 1O2, and the RGD peptide facilitates intracellular delivery. The small size, below 200 nm, as verified by scanning electron microscopy and differential light scattering measurements, of the architecture within the 1O2 diffusion length enables fast and efficient intracellular fluorescence switching by the tandem ultraviolet (UV)-visible or visible-near-infrared (NIR) photo-triggering. While the red emission and 1O2 generation by the porphyrin are continually turned on, the blue emission of coumarin is uncaged into 230-fold intensity enhancement by on-demand photo-triggering. The 1O2 production and release by the nanoarchitecture enable spectro-temporally controlled cell imaging and apoptotic cell death; the latter is verified from cytotoxic data under dark and phototriggering conditions. Furthermore, the bioimaging potential of the TCPP-based nanoarchitecture is examined in vivo in B6 mice.
中文翻译:

用于光动力治疗和生物成像的光敏剂-单线态氧传感器共轭二氧化硅纳米粒子
细胞内单线态氧 ( 1 O 2 ) 的生成和检测有助于优化光动力疗法 (PDT) 的结果。用于按需光触发1 O 2释放和生物成像的治疗诊断学具有改变 PDT 的巨大潜力。我们展示了超灵敏荧光开启传感器-敏化剂-RGD肽-二氧化硅纳米结构及其在单颗粒水平或活细胞中的1 O 2生成-释放-存储-传感特性。纳米结构中的传感器和敏化剂分别是氨甲基蒽(AMA)-香豆素二元体和卟啉或CdSe/ZnS量子点(QD)。二元体中的AMA通过分子内电子转移定量猝灭香豆素的荧光,卟啉或QD部分产生1 O 2 , RGD肽促进细胞内递送。通过扫描电子显微镜和微分光散射测量验证, 1 O 2扩散长度内的结构尺寸小,低于 200 nm,可通过串联紫外 (UV)-可见光或可见光-近光进行快速、高效的细胞内荧光切换。 - 红外 (NIR) 光触发。当卟啉的红光发射和1 O 2生成持续开启时,香豆素的蓝光发射通过按需光触发释放至 230 倍的强度增强。 纳米结构产生和释放的1 O 2能够实现光谱时间控制的细胞成像和细胞凋亡;后者通过黑暗和光触发条件下的细胞毒性数据得到验证。此外,基于 TCPP 的纳米结构的生物成像潜力在 B6 小鼠体内进行了体内检查。
更新日期:2023-12-14
中文翻译:

用于光动力治疗和生物成像的光敏剂-单线态氧传感器共轭二氧化硅纳米粒子
细胞内单线态氧 ( 1 O 2 ) 的生成和检测有助于优化光动力疗法 (PDT) 的结果。用于按需光触发1 O 2释放和生物成像的治疗诊断学具有改变 PDT 的巨大潜力。我们展示了超灵敏荧光开启传感器-敏化剂-RGD肽-二氧化硅纳米结构及其在单颗粒水平或活细胞中的1 O 2生成-释放-存储-传感特性。纳米结构中的传感器和敏化剂分别是氨甲基蒽(AMA)-香豆素二元体和卟啉或CdSe/ZnS量子点(QD)。二元体中的AMA通过分子内电子转移定量猝灭香豆素的荧光,卟啉或QD部分产生1 O 2 , RGD肽促进细胞内递送。通过扫描电子显微镜和微分光散射测量验证, 1 O 2扩散长度内的结构尺寸小,低于 200 nm,可通过串联紫外 (UV)-可见光或可见光-近光进行快速、高效的细胞内荧光切换。 - 红外 (NIR) 光触发。当卟啉的红光发射和1 O 2生成持续开启时,香豆素的蓝光发射通过按需光触发释放至 230 倍的强度增强。 纳米结构产生和释放的1 O 2能够实现光谱时间控制的细胞成像和细胞凋亡;后者通过黑暗和光触发条件下的细胞毒性数据得到验证。此外,基于 TCPP 的纳米结构的生物成像潜力在 B6 小鼠体内进行了体内检查。















































 京公网安备 11010802027423号
京公网安备 11010802027423号