当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of (Aza)fulvenallene, Cyanocyclopentadiene, and (Aza)fluorenes in the Thermal Rearrangements of Indazoles, Azaindazoles, and Homoquinolinic Anhydride
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-12-12 , DOI: 10.1021/acs.joc.3c02289 M Saeed Mirzaei 1 , Curt Wentrup 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-12-12 , DOI: 10.1021/acs.joc.3c02289 M Saeed Mirzaei 1 , Curt Wentrup 2
Affiliation
|
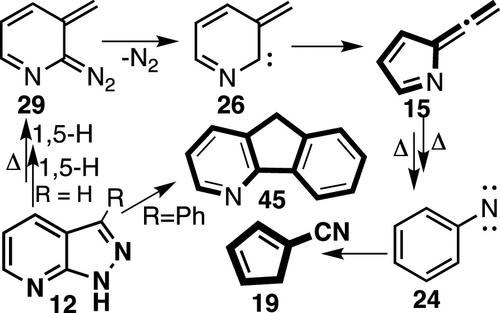
Flash vacuum pyrolysis (FVP) of pyrazoles and indazoles constitutes a valuable route to carbenes and nitrenes. In this study, we employed M062X and CCSD(T) calculations to provide a mechanistic rationale for the formation of fulvenallene and fluorenes from indazoles and the corresponding formation of azafulvenallene 15, cyanocyclopentadiene 19, and azafluorenes, e.g. 45, from azaindazoles, e.g. 12, and from homoquinolinic anhydride. The results reveal the importance of initial tautomerization in the pyrazole moiety of 7-azaindazole 12, which drives the mechanism toward 2-diazo-3-methylene-2,3-dihydropyridine 29 and hence 3-methylene-2,3-dihydropyridin-2-ylidene 26, followed by Wolff-type ring contraction to 1-azafulvenallene 15. This path has a calculated activation energy ∼10 kcal/mol lower than that for an alternate route involving ring opening to 3-diazomethylpyridine, dediazotization, and rearrangement of 3-pyridylcarbene to azacycloheptatetraene and phenylnitrene 24. FVP of 2,5-diphenyltetrazoles and phenyl(pyridyl)tetrazoles leads to nitrile imines, which cyclize to 3-phenylindazoles and -azaindazoles. Nitrogen elimination from these (aza) indazoles results in the formation of (aza) fluorenes, for which two alternate mechanisms are described: route A by rearrangement of (aza) indazoles to diazo(aza)cyclohexadienes and (aza)cyclohexadienylidenes and route B by rearrangement to diaryldiazomethanes and diarylcarbenes. Both paths are energetically feasible, but path A is preferred and corresponds to the azafluorenes obtained experimentally.
中文翻译:

吲唑、氮杂吲唑和高喹啉酸酐热重排中 (氮杂) 富文烯、氰基环戊二烯和 (氮杂) 芴的形成
吡唑和吲唑的闪蒸真空热解(FVP)是生产卡宾和氮宾的重要途径。在本研究中,我们采用 M062X 和 CCSD(T) 计算,为从吲唑形成富文烯和芴以及从氮杂吲唑(如12 )相应形成氮杂富烯15 、氰基环戊二烯19和氮杂芴(如45 )提供了机理原理。和来自高喹啉酸酐。结果揭示了 7-氮杂吲唑12的吡唑部分中初始互变异构化的重要性,这推动了机制向 2-重氮-3-亚甲基-2,3-二氢吡啶29发展,从而形成 3-亚甲基-2,3-二氢吡啶-2 -亚基26 ,随后沃尔夫型环收缩为 1-氮杂富烯15 。该路径的计算活化能比涉及 3-重氮甲基吡啶开环、脱重氮化以及 3-吡啶基卡宾重排为氮杂环庚四烯和苯基氮烯的替代路径低约 10 kcal/mol 24 。 2,5-二苯基四唑和苯基(吡啶基)四唑的FVP产生腈亚胺,其环化为3-苯基吲唑和氮杂吲唑。从这些(氮杂)吲唑中消除氮会导致(氮杂)芴的形成,对此描述了两种替代机制:通过(氮杂)吲唑重排为重氮(氮杂)环己二烯和(氮杂)亚环己二烯的路线A和通过以下路线B:重排为二芳基重氮甲烷和二芳基卡宾。两条路径在能量上都是可行的,但路径A是优选的并且对应于通过实验获得的氮杂芴。
更新日期:2023-12-12
中文翻译:

吲唑、氮杂吲唑和高喹啉酸酐热重排中 (氮杂) 富文烯、氰基环戊二烯和 (氮杂) 芴的形成
吡唑和吲唑的闪蒸真空热解(FVP)是生产卡宾和氮宾的重要途径。在本研究中,我们采用 M062X 和 CCSD(T) 计算,为从吲唑形成富文烯和芴以及从氮杂吲唑(如12 )相应形成氮杂富烯15 、氰基环戊二烯19和氮杂芴(如45 )提供了机理原理。和来自高喹啉酸酐。结果揭示了 7-氮杂吲唑12的吡唑部分中初始互变异构化的重要性,这推动了机制向 2-重氮-3-亚甲基-2,3-二氢吡啶29发展,从而形成 3-亚甲基-2,3-二氢吡啶-2 -亚基26 ,随后沃尔夫型环收缩为 1-氮杂富烯15 。该路径的计算活化能比涉及 3-重氮甲基吡啶开环、脱重氮化以及 3-吡啶基卡宾重排为氮杂环庚四烯和苯基氮烯的替代路径低约 10 kcal/mol 24 。 2,5-二苯基四唑和苯基(吡啶基)四唑的FVP产生腈亚胺,其环化为3-苯基吲唑和氮杂吲唑。从这些(氮杂)吲唑中消除氮会导致(氮杂)芴的形成,对此描述了两种替代机制:通过(氮杂)吲唑重排为重氮(氮杂)环己二烯和(氮杂)亚环己二烯的路线A和通过以下路线B:重排为二芳基重氮甲烷和二芳基卡宾。两条路径在能量上都是可行的,但路径A是优选的并且对应于通过实验获得的氮杂芴。

































 京公网安备 11010802027423号
京公网安备 11010802027423号