Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial Organization and Forces Arising from Epithelial–Cancerous Monolayer Interactions
ACS Nano ( IF 15.8 ) Pub Date : 2023-12-13 , DOI: 10.1021/acsnano.3c03990 Liu-Yuan Guan 1 , Shao-Zhen Lin 1 , Peng-Cheng Chen 1 , Jian-Qing Lv 1 , Bo Li 1 , Xi-Qiao Feng 1
ACS Nano ( IF 15.8 ) Pub Date : 2023-12-13 , DOI: 10.1021/acsnano.3c03990 Liu-Yuan Guan 1 , Shao-Zhen Lin 1 , Peng-Cheng Chen 1 , Jian-Qing Lv 1 , Bo Li 1 , Xi-Qiao Feng 1
Affiliation
|
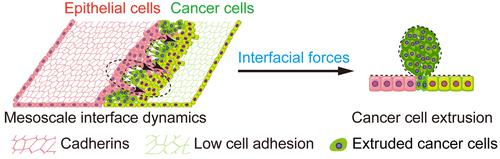
The interfacial interactions between epithelia and cancer cells have profound relevance for tumor development and metastasis. Through monolayer confrontation of MCF10A (nontumorigenic human breast epithelial cells) and MDA-MB-231 (human epithelial breast cancer cells) cells, we investigate the epithelial–cancerous interfacial interactions at the tissue level. We show that the monolayer interaction leads to competitive interfacial morphodynamics and drives an intricate spatial organization of MCF10A cells into multicellular finger-like structures, which further branch into multiple subfinger-like structures. These hierarchical interfacial structures penetrate the cancer monolayer and can spontaneously segregate or even envelop cancer cell clusters, consistent with our theoretical prediction. By tracking the substrate displacements via embedded fluorescent nanobeads and implementing nanomechanical modeling that combines atomic force microscopy and finite element simulations, we computed mechanical force patterns, including traction forces and monolayer stresses, caused by the monolayer interaction. It is found that the heterogeneous mechanical forces accumulated in the monolayers are able to squeeze cancer cells, leading to three-dimensional interfacial bulges or cell extrusion, initiating the p53 apoptosis signaling pathways of cancer cells. We reveal that intercellular E-cadherin and P-cadherin of epithelial cells differentially regulate the interfacial organization including migration speed, directionality, spatial correlation, F-actin alignment, and subcellular protrusions of MCF10A cells; whereas E-cadherin governs interfacial geometry that is relevant to force localization and cancer cell extrusion, P-cadherin maintains interfacial integrity that enables long-range force transmission. Our findings suggest that the collaborative molecular and mechanical behaviors are crucial for preventing epithelial tissues from undergoing tumor invasion.
中文翻译:

上皮-癌单层相互作用产生的界面组织和力
上皮细胞和癌细胞之间的界面相互作用与肿瘤的发展和转移具有深远的相关性。通过 MCF10A(非致瘤性人乳腺上皮细胞)和 MDA-MB-231(人上皮性乳腺癌细胞)细胞的单层对抗,我们研究了组织水平上皮癌界面相互作用。我们表明,单层相互作用导致竞争性界面形态动力学,并驱动 MCF10A 细胞复杂的空间组织形成多细胞指状结构,进一步分支成多个亚指状结构。这些分层界面结构穿透癌症单层,可以自发分离甚至包裹癌细胞簇,这与我们的理论预测一致。通过嵌入荧光纳米珠跟踪基板位移并实施结合原子力显微镜和有限元模拟的纳米力学建模,我们计算了由单层相互作用引起的机械力模式,包括牵引力和单层应力。研究发现,单层细胞中积累的异质机械力能够挤压癌细胞,导致三维界面凸起或细胞挤出,启动癌细胞的p53凋亡信号通路。 我们揭示了上皮细胞的细胞间E-钙粘蛋白和P-钙粘蛋白差异性地调节MCF10A细胞的界面组织,包括迁移速度、方向性、空间相关性、F-肌动蛋白排列和亚细胞突起; E-钙粘蛋白控制与力定位和癌细胞挤出相关的界面几何形状,而 P-钙粘蛋白则维持界面完整性,从而实现长距离力传递。我们的研究结果表明,协同分子和机械行为对于防止上皮组织遭受肿瘤侵袭至关重要。
更新日期:2023-12-13
中文翻译:

上皮-癌单层相互作用产生的界面组织和力
上皮细胞和癌细胞之间的界面相互作用与肿瘤的发展和转移具有深远的相关性。通过 MCF10A(非致瘤性人乳腺上皮细胞)和 MDA-MB-231(人上皮性乳腺癌细胞)细胞的单层对抗,我们研究了组织水平上皮癌界面相互作用。我们表明,单层相互作用导致竞争性界面形态动力学,并驱动 MCF10A 细胞复杂的空间组织形成多细胞指状结构,进一步分支成多个亚指状结构。这些分层界面结构穿透癌症单层,可以自发分离甚至包裹癌细胞簇,这与我们的理论预测一致。通过嵌入荧光纳米珠跟踪基板位移并实施结合原子力显微镜和有限元模拟的纳米力学建模,我们计算了由单层相互作用引起的机械力模式,包括牵引力和单层应力。研究发现,单层细胞中积累的异质机械力能够挤压癌细胞,导致三维界面凸起或细胞挤出,启动癌细胞的p53凋亡信号通路。 我们揭示了上皮细胞的细胞间E-钙粘蛋白和P-钙粘蛋白差异性地调节MCF10A细胞的界面组织,包括迁移速度、方向性、空间相关性、F-肌动蛋白排列和亚细胞突起; E-钙粘蛋白控制与力定位和癌细胞挤出相关的界面几何形状,而 P-钙粘蛋白则维持界面完整性,从而实现长距离力传递。我们的研究结果表明,协同分子和机械行为对于防止上皮组织遭受肿瘤侵袭至关重要。






























 京公网安备 11010802027423号
京公网安备 11010802027423号