Chemical Engineering Science ( IF 4.1 ) Pub Date : 2023-12-12 , DOI: 10.1016/j.ces.2023.119641 Rajesh Rajendiran , Putrakumar Balla , Ravi Balaga , Manickam Selvaraj , Vijayanand Perupogu , Murugesan Sepperumal , Ulla Lassi , Prem Kumar Seelam
|
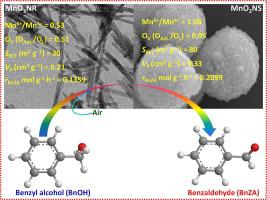
Understanding the relationship between the structure and the activity of the catalyst relies on the shape–controlled synthesis of nanostructures is crucial importance. The vapor phase oxidation of benzyl alcohol (BnOH) process over morphologically designed shape–selective manganese oxide nanorods (MnO2NR) and nanospheres (MnO2NS) catalysts are studied. The key catalytic properties are determined and demonstrated by various characterisation techniques such as P–XRD, BET, H2–TPR, O2–TPD, and Raman analysis. In addition, EDX and STEM–HRTEM microscopic analysis were carried out in better understanding the surface morphology, shape, and structure of the nanocatalysts. The prepared nanoporous MnO2NS catalyst enabled to generate more crystal defects, high surface area, strong reducing capacity, enhanced oxygen vacancies (Ov), and increased reactive surface oxygen species compared to nanorod shaped–MnO2NR catalyst. Significantly the rate of benzaldehyde (BnZA) formation in BnOH oxidation reaction over MnO2NS catalyst is ∼ 1.55 times higher than that of MnO2NR. Over MnO2NS creation of abundant O vacancies considerably improved the capacity of oxygen activation and redox ability. Thus, as a result, there are more active oxygen species which are mobile and reactive in accelerating the BnOH oxidation process.
中文翻译:

空气存在下气相苯甲醇 (BnOH) 氧化中 MnO2 形状依赖的催化活性
了解催化剂的结构和活性之间的关系依赖于纳米结构的形状控制合成至关重要。研究了在形态设计的形状选择性氧化锰纳米棒(MnO 2 NR)和纳米球(MnO 2 NS)催化剂上的苯甲醇(BnOH)气相氧化过程。关键的催化性能通过各种表征技术确定和证明,例如 P-XRD、BET、H 2 -TPR、O 2 -TPD 和拉曼分析。此外,还进行了 EDX 和 STEM-HRTEM 显微分析,以更好地了解纳米催化剂的表面形态、形状和结构。所制备的纳米多孔MnO 2 NS催化剂与相比,能够产生更多的晶体缺陷、高比表面积、强还原能力、增强的氧空位(O v )和增加的活性表面氧物种纳米棒状–MnO 2 NR催化剂。值得注意的是,MnO 2 NS 催化剂上 BnOH 氧化反应中苯甲醛 (BnZA) 的形成速率比 MnO 2 NR 高约 1.55 倍。 MnO 2 NS 产生丰富的 O 空位,大大提高了氧的活化能力和氧化还原能力。因此,有更多的活性氧物种,它们具有流动性和反应性,加速了 BnOH 氧化过程。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号