当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu2O facet controlled reactivity for peroxidase-like activity
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2023-11-28 , DOI: 10.1039/d3cy01399e
Shivanand Chettri , Liang-Ting Wu , Sagarmani Rasaily , Debesh Sharma , Bikram Gurung , Rajani Dewan , Sudarsan Tamang , Jyh-Chiang Jiang , Anand Pariyar
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2023-11-28 , DOI: 10.1039/d3cy01399e
Shivanand Chettri , Liang-Ting Wu , Sagarmani Rasaily , Debesh Sharma , Bikram Gurung , Rajani Dewan , Sudarsan Tamang , Jyh-Chiang Jiang , Anand Pariyar
|
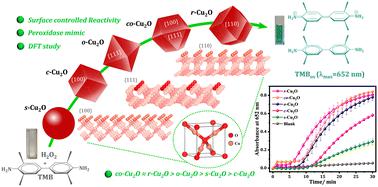
Replicating the enzymatic surface microenvironment in vitro is challenging, but constructing an analogous model could facilitate our understanding of surface effects and aid in developing an efficient bioinspired catalytic system. In this study, we generate five unique Cu2O morphologies: cubic (c-Cu2O), octahedral (o-Cu2O), rhombododecahedral (r-Cu2O), cuboctahedral (co-Cu2O), and spherical (s-Cu2O) with an average edge length between 0.59 and 0.61 μm. By precisely controlling crystal growth on distinct surface planes, with surface energies in the order γ{100} < γ{111} < γ{110}, we achieve a wide spectrum of shape variations during the evolution of these structures. The variations in surface morphology are a consequence of differences in the exposure of low-index facets, such as {100}, {111}, and {110}, leading to varying numbers of unsaturated copper sites at the surface. The peroxidase-like activity was investigated by altering the Cu2O surface structures in the reduction of H2O2 using chromogenic substrates like TMB/ABTS. The activity catalyzed by various Cu2O surfaces (r-Cu2O, o-Cu2O, and c-Cu2O) followed the typical Michaelis–Menten kinetics, with Km values ranging from 0.096 to 0.120 mM for TMB and 1.57 to 2.90 mM for H2O2 as substrates, respectively. Compared to native peroxidase enzymes (with Km values of 0.434 mM for TMB and 3.70 mM for H2O2 under identical conditions), these Cu2O catalysts exhibited a higher affinity. In general, the reactivity order observed was as follows: co-Cu2O ≈ r-Cu2O > o-Cu2O > c-Cu2O > s-Cu2O. Mechanistically, the H2O2 reduction on the surface produces hydroxyl radicals that undergo H-abstraction from TMB, where the latter was found to be the rate-determining step. Both kinetic and DFT studies have unveiled that the heightened reactivity of r-Cu2O can be attributed to its higher proportion of {110} planes, which contain a higher number of dangling (unsaturated) Cu atoms that facilitate H2O2 decomposition. Additionally, they exhibit sufficiently low energy barriers (TS2: 0.70 eV) that enable OH radicals to efficiently oxidize TMB molecules compared to other morphologies.
中文翻译:

Cu2O 面控制的过氧化物酶样活性的反应性
在体外复制酶表面微环境具有挑战性,但构建类似的模型可以促进我们对表面效应的理解,并有助于开发有效的仿生催化系统。在这项研究中,我们生成了五种独特的 Cu 2 O 形态:立方 (c-Cu 2 O)、八面体 (o-Cu 2 O)、菱形十二面体 (r-Cu 2 O)、立方八面体 (co-Cu 2 O) 和球形 (s-Cu 2 O),平均边长在 0.59 至 0.61 μm 之间。通过精确控制不同表面平面上的晶体生长,表面能的顺序为γ {100} < γ {111} < γ {110},我们在这些结构的演化过程中实现了广泛的形状变化。表面形态的变化是低折射率面(例如{100}、{111}和{110})暴露差异的结果,导致表面上不饱和铜位点的数量不同。通过使用TMB/ABTS 等显色底物还原H 2 O 2时改变Cu 2 O 表面结构来研究过氧化物酶样活性。各种 Cu 2 O 表面(r-Cu 2 O、o-Cu 2 O 和 c-Cu 2 O)催化的活性遵循典型的 Michaelis-Menten 动力学, TMB 和 TMB 的K m值范围为 0.096 至 0.120 mM H 2 O 2作为底物时分别为1.57 至2.90 mM。与天然过氧化物酶(在相同条件下,TMB 的K m值为 0.434 mM,H 2 O 2的 K m 值为 3.70 mM)相比,这些 Cu 2 O 催化剂表现出更高的亲和力。一般来说,观察到的反应顺序如下:co-Cu 2 O ≈ r-Cu 2 O > o-Cu 2 O > c-Cu 2 O > s-Cu 2 O。从机理上讲,H 2 O 2还原表面产生羟基自由基,该羟基自由基从 TMB 中夺氢,发现后者是速率决定步骤。动力学和 DFT 研究均表明,r-Cu 2 O 的较高反应性可归因于其较高比例的 {110} 平面,其中包含更多数量的悬挂(不饱和)Cu 原子,有利于 H 2 O 2分解。此外,与其他形态相比,它们表现出足够低的能垒(TS2:0.70 eV),使 OH 自由基能够有效氧化 TMB 分子。
更新日期:2023-11-28
中文翻译:

Cu2O 面控制的过氧化物酶样活性的反应性
在体外复制酶表面微环境具有挑战性,但构建类似的模型可以促进我们对表面效应的理解,并有助于开发有效的仿生催化系统。在这项研究中,我们生成了五种独特的 Cu 2 O 形态:立方 (c-Cu 2 O)、八面体 (o-Cu 2 O)、菱形十二面体 (r-Cu 2 O)、立方八面体 (co-Cu 2 O) 和球形 (s-Cu 2 O),平均边长在 0.59 至 0.61 μm 之间。通过精确控制不同表面平面上的晶体生长,表面能的顺序为γ {100} < γ {111} < γ {110},我们在这些结构的演化过程中实现了广泛的形状变化。表面形态的变化是低折射率面(例如{100}、{111}和{110})暴露差异的结果,导致表面上不饱和铜位点的数量不同。通过使用TMB/ABTS 等显色底物还原H 2 O 2时改变Cu 2 O 表面结构来研究过氧化物酶样活性。各种 Cu 2 O 表面(r-Cu 2 O、o-Cu 2 O 和 c-Cu 2 O)催化的活性遵循典型的 Michaelis-Menten 动力学, TMB 和 TMB 的K m值范围为 0.096 至 0.120 mM H 2 O 2作为底物时分别为1.57 至2.90 mM。与天然过氧化物酶(在相同条件下,TMB 的K m值为 0.434 mM,H 2 O 2的 K m 值为 3.70 mM)相比,这些 Cu 2 O 催化剂表现出更高的亲和力。一般来说,观察到的反应顺序如下:co-Cu 2 O ≈ r-Cu 2 O > o-Cu 2 O > c-Cu 2 O > s-Cu 2 O。从机理上讲,H 2 O 2还原表面产生羟基自由基,该羟基自由基从 TMB 中夺氢,发现后者是速率决定步骤。动力学和 DFT 研究均表明,r-Cu 2 O 的较高反应性可归因于其较高比例的 {110} 平面,其中包含更多数量的悬挂(不饱和)Cu 原子,有利于 H 2 O 2分解。此外,与其他形态相比,它们表现出足够低的能垒(TS2:0.70 eV),使 OH 自由基能够有效氧化 TMB 分子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号