当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alternative Routes to 4,6-O-Benzylidene β-Thioglycosides
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2023-12-11 , DOI: 10.1002/hlca.202300193 Luigi Panza 1 , Daniela Imperio 2 , Filippo Valloni 3
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2023-12-11 , DOI: 10.1002/hlca.202300193 Luigi Panza 1 , Daniela Imperio 2 , Filippo Valloni 3
Affiliation
|
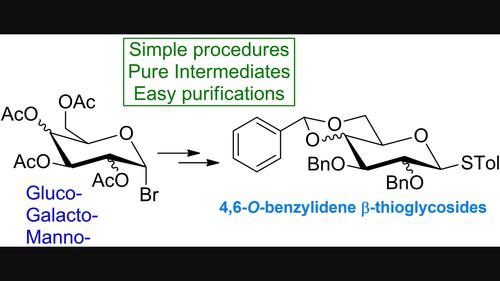
The synthesis of glycosides generally requires the use of building blocks that need to be readily prepared, avoiding tricky reaction steps and boring purifications. Thioglycosides represent key donor intermediates for their activation in glycosylation reactions and for their great stability. Moreover, the presence of a benzylidene moiety confers to the molecule a double advantage: it can influence the stereochemistry of the glycosylation reaction and can be selectively opened to generate different species having a free secondary hydroxyl group. Here, the preparation of p-Tolyl 1-thio-4,6-O-benzylidene-2,3-di-O-benzyl-β-d-pyranoses of glucose, galactose, and mannose are described. The global procedure involved the exploitation of simple reactions and crystallization techniques, having a chromatographic purification for the last step only.
中文翻译:

4,6-O-亚苄基 β-硫代糖苷的替代途径
糖苷的合成通常需要使用易于制备的结构单元,避免棘手的反应步骤和无聊的纯化。硫代糖苷是其在糖基化反应中的活化和高度稳定性的关键供体中间体。此外,亚苄基部分的存在赋予该分子双重优势:它可以影响糖基化反应的立体化学,并且可以选择性地打开以产生具有游离仲羟基的不同物质。这里,描述了葡萄糖、半乳糖和甘露糖的对甲苯基1-硫代-4,6- O-亚苄基-2,3-二-O-苄基-β- d-吡喃糖的制备。整体程序涉及简单反应和结晶技术的开发,仅在最后一步进行色谱纯化。
更新日期:2023-12-11
中文翻译:

4,6-O-亚苄基 β-硫代糖苷的替代途径
糖苷的合成通常需要使用易于制备的结构单元,避免棘手的反应步骤和无聊的纯化。硫代糖苷是其在糖基化反应中的活化和高度稳定性的关键供体中间体。此外,亚苄基部分的存在赋予该分子双重优势:它可以影响糖基化反应的立体化学,并且可以选择性地打开以产生具有游离仲羟基的不同物质。这里,描述了葡萄糖、半乳糖和甘露糖的对甲苯基1-硫代-4,6- O-亚苄基-2,3-二-O-苄基-β- d-吡喃糖的制备。整体程序涉及简单反应和结晶技术的开发,仅在最后一步进行色谱纯化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号