当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Native Peptide Cyclization, Sequential Chemoselective Amidation in Water
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-11 , DOI: 10.1021/jacs.3c10341 Huan Chen 1 , Qiang Zhang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-11 , DOI: 10.1021/jacs.3c10341 Huan Chen 1 , Qiang Zhang 1
Affiliation
|
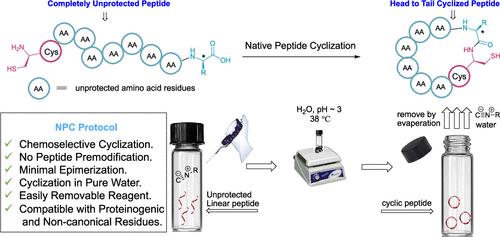
Chemical synthesis offers robust tactics for structural alterations of peptides and proteins. It remains a labor-intensive and complex process due to the challenges in selectively modifying diverse amino acid side chains and termini. Direct α-peptide ligation without premodification is a significant hurdle, especially when aiming to include all proteinogenic amino acids at the ligation site. We introduce Native Peptide Cyclization (NPC), a chemoselective method enabling intramolecular peptidyl ligation without the need for premodification. NPC cyclizes unprotected linear peptides through controlled, sequential C- and N-terminal activation via pH modulation. Water-based NPC simplifies peptide ligation, easing the labor-intensive nature of peptide synthesis, aiding efficient cyclic peptide preparation and enabling cost-effective macrocycle-based therapeutics.
中文翻译:

水中天然肽环化、连续化学选择性酰胺化
化学合成为肽和蛋白质的结构改变提供了强有力的策略。由于选择性修饰不同氨基酸侧链和末端的挑战,它仍然是一个劳动密集型且复杂的过程。未经预修饰的直接 α 肽连接是一个重大障碍,特别是当旨在包含连接位点的所有蛋白氨基酸时。我们引入天然肽环化 (NPC),这是一种无需预修饰即可进行分子内肽基连接的化学选择性方法。 NPC 通过 pH 调节控制、连续的 C 端和 N 端激活来环化未受保护的线性肽。水基 NPC 简化了肽连接,减轻了肽合成的劳动密集型性质,有助于有效的环肽制备并实现经济有效的基于大环的治疗。
更新日期:2023-12-11
中文翻译:

水中天然肽环化、连续化学选择性酰胺化
化学合成为肽和蛋白质的结构改变提供了强有力的策略。由于选择性修饰不同氨基酸侧链和末端的挑战,它仍然是一个劳动密集型且复杂的过程。未经预修饰的直接 α 肽连接是一个重大障碍,特别是当旨在包含连接位点的所有蛋白氨基酸时。我们引入天然肽环化 (NPC),这是一种无需预修饰即可进行分子内肽基连接的化学选择性方法。 NPC 通过 pH 调节控制、连续的 C 端和 N 端激活来环化未受保护的线性肽。水基 NPC 简化了肽连接,减轻了肽合成的劳动密集型性质,有助于有效的环肽制备并实现经济有效的基于大环的治疗。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号