当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, Antiacetylcholinesterase Activity, and Molecular Dynamics Simulation of Aporphine–benzylpyridinium Conjugates
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2023-12-08 , DOI: 10.1021/acsmedchemlett.3c00467 Nisachon Khunnawutmanotham 1 , Pichjira Sooknual 1 , Paratchata Batsomboon 2 , Poonsakdi Ploypradith 2, 3 , Nitirat Chimnoi 4 , Apinya Patigo 5 , Patchreenart Saparpakorn 5 , Supanna Techasakul 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2023-12-08 , DOI: 10.1021/acsmedchemlett.3c00467 Nisachon Khunnawutmanotham 1 , Pichjira Sooknual 1 , Paratchata Batsomboon 2 , Poonsakdi Ploypradith 2, 3 , Nitirat Chimnoi 4 , Apinya Patigo 5 , Patchreenart Saparpakorn 5 , Supanna Techasakul 1
Affiliation
|
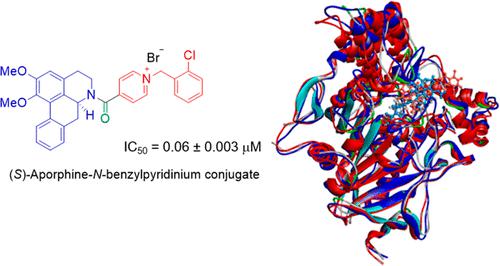
A series of aporphines conjugated with an N-benzylpyridinium moiety through an amide-bond linkage were synthesized and evaluated for their acetylcholinesterase (AChE) inhibitory activity. The conjugation of the N-benzylpyridinium group significantly enhanced the AChE inhibitory activity of the core aporphine. The halogen substituents on the benzyl group affected the activity of the conjugates. Both (S)- and (R)-enantiomers of three conjugates with low IC50 values were synthesized and evaluated for their activities. All (S)-enantiomers exhibited higher activity than the corresponding (R)-enantiomers. The (S)-enantiomer of 2-chlorobenzylpyridinium-containing aporphine was the most potent inhibitor in this study with an IC50 value of 0.06 ± 0.003 μM. Molecular dynamics simulation analysis revealed that both enantiomers can interact with the AChE binding site, whereas the (S)-enantiomer possessed slightly stronger interaction than the (R)-enantiomer, presumably because of their different orientations, as evidenced by molecular docking. The N-benzylpyridinium dehydroaporphine conjugates were also synthesized but were less active than the corresponding aporphine conjugates.
中文翻译:

阿朴啡-苄基吡啶结合物的合成、抗乙酰胆碱酯酶活性和分子动力学模拟
合成了一系列通过酰胺键与N-苄基吡啶部分缀合的阿朴啡,并评估了它们的乙酰胆碱酯酶 (AChE) 抑制活性。N-苄基吡啶鎓基团的缀合显着增强了核心阿朴啡的AChE抑制活性。苄基上的卤素取代基影响缀合物的活性。合成了三种具有低IC 50值的缀合物的( S )-和( R )-对映体并评估了它们的活性。所有 ( S )-对映体均表现出比相应的 ( R )-对映体更高的活性。含 2-氯苄基吡啶鎓的( S )-对映体阿朴啡是本研究中最有效的抑制剂,IC 50值为 0.06 ± 0.003 μM。分子动力学模拟分析表明,两种对映体都能与AChE结合位点相互作用,而( S )-对映体的相互作用略强于( R )-对映体,这可能是因为它们的方向不同,分子对接证明了这一点。还合成了N-苄基吡啶鎓脱氢阿朴啡缀合物,但其活性低于相应的阿朴啡缀合物。
更新日期:2023-12-08
中文翻译:

阿朴啡-苄基吡啶结合物的合成、抗乙酰胆碱酯酶活性和分子动力学模拟
合成了一系列通过酰胺键与N-苄基吡啶部分缀合的阿朴啡,并评估了它们的乙酰胆碱酯酶 (AChE) 抑制活性。N-苄基吡啶鎓基团的缀合显着增强了核心阿朴啡的AChE抑制活性。苄基上的卤素取代基影响缀合物的活性。合成了三种具有低IC 50值的缀合物的( S )-和( R )-对映体并评估了它们的活性。所有 ( S )-对映体均表现出比相应的 ( R )-对映体更高的活性。含 2-氯苄基吡啶鎓的( S )-对映体阿朴啡是本研究中最有效的抑制剂,IC 50值为 0.06 ± 0.003 μM。分子动力学模拟分析表明,两种对映体都能与AChE结合位点相互作用,而( S )-对映体的相互作用略强于( R )-对映体,这可能是因为它们的方向不同,分子对接证明了这一点。还合成了N-苄基吡啶鎓脱氢阿朴啡缀合物,但其活性低于相应的阿朴啡缀合物。







































 京公网安备 11010802027423号
京公网安备 11010802027423号