当前位置:
X-MOL 学术
›
J. Environ. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation of benzophenone-8 in UV/oxidation processes: Comparison of UV/H2O2, UV/persulfate, UV/chlorine processes
Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2023-12-09 , DOI: 10.1016/j.jece.2023.111623 Young-Min Lee , Gowoon Lee , Taeyeon Kim , Kyung-Duk Zoh
Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2023-12-09 , DOI: 10.1016/j.jece.2023.111623 Young-Min Lee , Gowoon Lee , Taeyeon Kim , Kyung-Duk Zoh
|
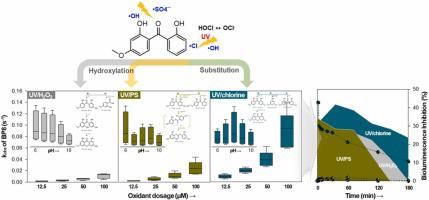
Benzopenone-8 (BP8) is an ultraviolet (UV) filter commonly used in sunscreen and plastic products. BP8 can cause acute and chronic toxicity in living organisms because it is biologically toxic and bioaccumulates. Advanced oxidation processes (AOPs) are widely used to treat contaminated water using powerful radical species. In this study, we compared the degradation kinetics and mechanisms of BP8 under UV/HO, UV/persulfate (PS), and UV/chlorine processes. BP8 reacts quickly with reactive oxidants following pseudo-first-order kinetics. At an oxidant concentration of 12.5 μM within the pH range of 6–10, the degradation rate constant () was the highest for the UV/chlorine ((5.29–14.2) ×10 s), followed by UV/PS ((1.38–2.90) ×10 s) and UV/HO ((0.79–2.41) ×10 s). increased linearly with increasing oxidant dosage, with the highest value at pH 6 in the UV/HO and UV/PS and pH 8 in the UV/chlorine process. At the pH with the highest , hydroxyl radicals (∙OH), sulfate radicals (∙SO), and reactive chlorine species played significant roles in removing 99.7 %, 82.3 %, and 56.2 % of BP8 in the UV/HO, UV/PS, and UV/chlorine processes, respectively. Humic acids significantly inhibited BP8 degradation, whereas NO ions promoted BP8 degradation in UV/HO process, NO and Cl ions promoted BP8 degradation in UV/PS process and Br ions promoted BP8 degradation in UV/chlorine process. The electricity consumption (EE/O) in various water matrices was in the order of UV/HO > UV/PS >> UV/chlorine process; however, the chlorinated transformation products (TPs) produced in the UV/chlorine process were more toxic than the hydroxylated TPs from the UV/HO and UV/PS processes, implying that the UV/PS process was most suitable for removing BP8 while controlling the formation of toxic products.
中文翻译:

UV/氧化过程中二苯甲酮-8 的降解:UV/H2O2、UV/过硫酸盐、UV/氯过程的比较
Benzopenone-8 (BP8) 是一种紫外线 (UV) 过滤剂,常用于防晒霜和塑料产品。BP8 具有生物毒性并具有生物蓄积性,因此可对生物体造成急性和慢性毒性。高级氧化工艺 (AOP) 广泛用于使用强自由基物质处理污染水。在本研究中,我们比较了 BP8 在 UV/H2O、UV/过硫酸盐 (PS) 和 UV/氯过程下的降解动力学和机制。BP8 按照伪一级动力学与活性氧化剂快速反应。在氧化剂浓度为 12.5 μM、pH 6-10 范围内,UV/氯 ((5.29-14.2) ×10 s) 的降解速率常数 () 最高,其次是 UV/PS ((1.38- 2.90) ×10 s) 和 UV/H2O ((0.79–2.41) ×10 s)。随着氧化剂用量的增加而线性增加,在 UV/H2O 和 UV/PS 中的 pH 6 时达到最高值,在 UV/氯工艺中的 pH 8 时达到最高值。在 pH 值最高时,羟基自由基 (∙OH)、硫酸根 (∙SO) 和活性氯在 UV/H2O、UV/PS 中去除 99.7 %、82.3 % 和 56.2 % BP8 方面发挥了重要作用、 和 UV/氯工艺。腐殖酸显着抑制BP8降解,而NO离子在UV/H2O过程中促进BP8降解,NO和Cl离子在UV/PS过程中促进BP8降解,Br离子在UV/氯过程中促进BP8降解。各种水基体的耗电量(EE/O)顺序为UV/H2O > UV/PS >> UV/氯工艺;然而,UV/氯工艺中产生的氯化转化产物(TPs)比UV/H2O和UV/PS工艺中产生的羟基化TPs毒性更大,这意味着UV/PS工艺最适合去除BP8,同时控制有毒产物的形成。
更新日期:2023-12-09
中文翻译:

UV/氧化过程中二苯甲酮-8 的降解:UV/H2O2、UV/过硫酸盐、UV/氯过程的比较
Benzopenone-8 (BP8) 是一种紫外线 (UV) 过滤剂,常用于防晒霜和塑料产品。BP8 具有生物毒性并具有生物蓄积性,因此可对生物体造成急性和慢性毒性。高级氧化工艺 (AOP) 广泛用于使用强自由基物质处理污染水。在本研究中,我们比较了 BP8 在 UV/H2O、UV/过硫酸盐 (PS) 和 UV/氯过程下的降解动力学和机制。BP8 按照伪一级动力学与活性氧化剂快速反应。在氧化剂浓度为 12.5 μM、pH 6-10 范围内,UV/氯 ((5.29-14.2) ×10 s) 的降解速率常数 () 最高,其次是 UV/PS ((1.38- 2.90) ×10 s) 和 UV/H2O ((0.79–2.41) ×10 s)。随着氧化剂用量的增加而线性增加,在 UV/H2O 和 UV/PS 中的 pH 6 时达到最高值,在 UV/氯工艺中的 pH 8 时达到最高值。在 pH 值最高时,羟基自由基 (∙OH)、硫酸根 (∙SO) 和活性氯在 UV/H2O、UV/PS 中去除 99.7 %、82.3 % 和 56.2 % BP8 方面发挥了重要作用、 和 UV/氯工艺。腐殖酸显着抑制BP8降解,而NO离子在UV/H2O过程中促进BP8降解,NO和Cl离子在UV/PS过程中促进BP8降解,Br离子在UV/氯过程中促进BP8降解。各种水基体的耗电量(EE/O)顺序为UV/H2O > UV/PS >> UV/氯工艺;然而,UV/氯工艺中产生的氯化转化产物(TPs)比UV/H2O和UV/PS工艺中产生的羟基化TPs毒性更大,这意味着UV/PS工艺最适合去除BP8,同时控制有毒产物的形成。































 京公网安备 11010802027423号
京公网安备 11010802027423号