European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-12-09 , DOI: 10.1016/j.ejmech.2023.116036 Pierre Francotte 1 , Yasmin Bay 2 , Eric Goffin 1 , Thomas Colson 1 , Cindy Lesenfants 1 , Jerzy Dorosz 2 , Saara Laulumaa 2 , Pierre Fraikin 1 , Pascal de Tullio 1 , Caroline Beaufour 3 , Iuliana Botez 3 , Darryl S Pickering 2 , Karla Frydenvang 2 , Laurence Danober 3 , Anders Skov Kristensen 2 , Jette Sandholm Kastrup 2 , Bernard Pirotte 1
|
The synthesis and biological evaluation on AMPA and kainate receptors of new examples of 3,4-dihydro-2H-1,2,4-thieno[3,2-e]-1,2,4-thiadiazine 1,1-dioxides is described.
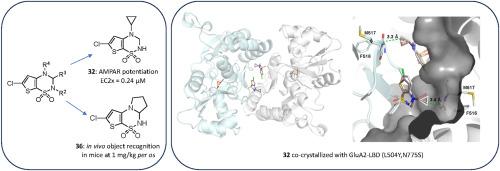
The introduction of a cyclopropyl chain instead of an ethyl chain at the 4-position of the thiadiazine ring was found to dramatically improve the potentiator activity on AMPA receptors, with compound 32 (BPAM395) expressing in vitro activity on AMPARs (EC2x = 0.24 μM) close to that of the reference 4-cyclopropyl-substituted benzothiadiazine dioxide 10 (BPAM344).
Interestingly, the 4-allyl-substituted thienothiadiazine dioxide 27 (BPAM307) emerged as the most promising compound on kainate receptors being a more effective potentiator than the 4-cyclopropyl-substituted thienothiadiazine dioxide 32 and supporting the view that the 4-allyl substitution of the thiadiazine ring could be more favorable than the 4-cyclopropyl substitution to induce marked activity on kainate receptors versus AMPA receptors.
The thieno-analogue 36 (BPAM279) of the clinically tested S18986 (11) was selected for in vivo evaluation in mice as a cognitive enhancer due to a safer profile than 32 after massive per os drug administration. Compound 36 was found to increase the cognition performance in mice at low doses (1 mg/kg) per os suggesting that the compound was well absorbed after oral administration and able to reach the central nervous system.
Finally, compound 32 was selected for co-crystallization with the GluA2-LBD (L504Y,N775S) and glutamate to examine the binding mode of thienothiadiazine dioxides within the allosteric binding site of the AMPA receptor. At the allosteric site, this compound established similar interactions as the previously reported BTD-type AMPA receptor modulators.
中文翻译:

在寻找新的 AMPA 和红藻氨酸受体正变构调节剂中探索二氧化噻吩并噻二嗪作为苯并和吡啶并噻二嗪二氧化物的等排类似物
3,4-二氢-2 H -1,2,4-噻吩并[3,2- e ]-1,2,4-噻二嗪1,1-二氧化物新实例的合成及AMPA和红藻氨酸受体的生物学评价被描述。
研究发现,在噻二嗪环的 4 位引入环丙基链而不是乙基链可显着提高 AMPA 受体的增效剂活性,化合物32 (BPAM395) 在 AMPAR 上表现出体外活性(EC2x = 0.24 μM)接近参考品 4-环丙基取代的苯并噻二嗪二氧化物10 (BPAM344)。
有趣的是,4-烯丙基取代的噻吩并噻二嗪二氧化27 (BPAM307) 成为红藻氨酸受体上最有前途的化合物,是比 4-环丙基取代的噻吩并噻二嗪二氧化32更有效的增效剂,并支持了这样的观点:4-烯丙基取代的噻吩并噻二嗪二氧化与 AMPA 受体相比,噻二嗪环可能比 4-环丙基取代更有利地诱导红藻氨酸受体显着活性。
选择临床测试的 S18986 ( 11 ) 的噻吩类似物36 (BPAM279) 作为认知增强剂在小鼠体内进行评估,因为在大量口服药物给药后比32更安全。研究发现,口服低剂量(1 mg/kg)化合物36可提高小鼠的认知能力,表明该化合物口服后吸收良好,并且能够到达中枢神经系统。
最后,选择化合物32与 GluA2-LBD (L504Y,N775S) 和谷氨酸共结晶,以检查二氧化噻吩并噻二嗪在 AMPA 受体变构结合位点内的结合模式。在变构位点,该化合物建立了与之前报道的 BTD 型 AMPA 受体调节剂类似的相互作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号