Solid State Sciences ( IF 3.4 ) Pub Date : 2023-12-10 , DOI: 10.1016/j.solidstatesciences.2023.107403
Mahsa Foroughian , Tiffany M.Smith Pellizzeri , Colin D. McMillen , Kimberly Ivey , Joseph W. Kolis
|
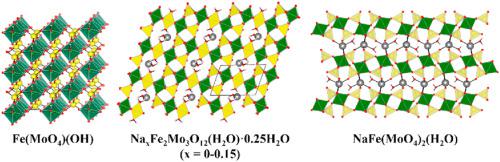
Several new iron molybdates have been synthesized under hydrothermal conditions at relatively low temperatures (200 °C) to stabilize the Fe3+ oxidation state. The mineralizers are all sodium ion based and three new phases were isolated as high quality single crystals from various reaction stoichiometries, Fe(MoO4)(OH) (I), Na0·15Fe2Mo3O12(H2O)·0.25H2O (IIa), and NaFe(MoO4)2(H2O) (III). The structures contain iron oxide octahedra arranged in chains (I), dimers (IIa), and isolated octahedra (III), that are linked by various molybdate oxyanions to form 3-D framework (I), 3-D framework with channels (IIa), and 2-D sheets in their iron molybdate motifs. Both I and III are lightly colored materials containing valence precise Fe3+, whereas IIa contains partially occupied Na + ions in columns within the lattice leading to concomitant valence mixing of Fe2+/Fe3+ with a corresponding darker color to the crystal. If sodium ions are not included in the synthesis and instead replaced by smaller Li + ions in the reaction, there is no alkali metal incorporation in the lattice and the resultant compound Fe2Mo3O12(H2O)·0.25H2O (IIb) is formed. This is isostructural to IIa but without the partially occupied Na + ions in the channels which makes it another valence precise Fe3+ compound that is nearly colorless as expected since all transtions are spin forbidden in the high spin Fe3+ case. The role of carbonate as a mineralizer was examined since the reaction is highly acidic (pH ∼ 1) in the absence of carbonate, and this does not lead to satisfactory product formation. The carbonate mineralizer increases the pH to 6–6.5, and this pH is maintained throughout the reaction, which does lead to high quality crystal products. Despite its absence in any of the products, the carbonate mineralizer plays an additional important (albeit unknown) role in the synthesis, because if simple hydroxide is used to increase the pH to 6–6.5, the resultant products are much lower in quality and yield.
中文翻译:

碳酸钠溶液水热合成钼酸铁及其结构
在相对较低的温度 (200 °C) 的水热条件下合成了几种新型钼酸铁,以稳定 Fe3+ 氧化< /span>)4) 和 NaFe(MoOIIaO ( 2O)·0.25H2(H12O3Mo2Fe0·15)、NaI)(OH) ( 4 状态。矿化剂都是钠离子基的,并且从各种反应化学计量中分离出三个新相作为高质量单晶,Fe(MoO(H2O) (III都是浅色材料,含有价态精确的Fe/Fe2 化合物,该化合物几乎如预期无色,因为所有跃迁在高自旋 Fe 中都是自旋禁止的 案例。研究了碳酸盐作为矿化剂的作用,因为在没有碳酸盐的情况下反应呈高酸性(pH ~ 1),这不会导致令人满意的产物形成。碳酸盐矿化剂将 pH 值提高到 6-6.5,并且在整个反应过程中保持该 pH 值,这确实产生了高质量的晶体产品。尽管任何产品中都不存在碳酸盐矿化剂,但碳酸盐矿化剂在合成中起着额外重要(尽管未知)的作用,因为如果使用简单的氢氧化物将 pH 值提高到 6-6.5,所得产品的质量和产量会大大降低.3+3+ 离子通道使其成为另一种价态精确的 Fe+ 同构,但不含部分占据的 Na IIa)。这与 IIbO(O)·0.25H2(H12O3Mo2 离子取代,则晶格中不会掺入碱金属,并且所得化合物 Fe+ 具有与晶体相应的较深颜色。如果合成中不包含钠离子,而是在反应中被较小的 Li 3+2+ 晶格内柱中的离子导致 Fe 伴随价态混合+ 包含部分占据的 Na IIa,而 3+III和I) 以及铁中的 2-D 板材钼酸盐图案。 IIa)、带通道的 3-D 框架 (I),它们通过各种钼酸根氧阴离子连接形成3-D框架(III)和孤立的八面体(IIa)、二聚体 (I)。该结构包含呈链状排列的氧化铁八面体 (































 京公网安备 11010802027423号
京公网安备 11010802027423号