European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2023-12-08 , DOI: 10.1016/j.ejps.2023.106669 Olivia Campagne 1 , Jie Huang 2 , Tong Lin 2 , Wilburn E Reddick 3 , Nicholas S Selvo 1 , Arzu Onar-Thomas 2 , Deborah Ward 1 , Giles Robinson 4 , Amar Gajjar 4 , Clinton F Stewart 1
|
Purpose
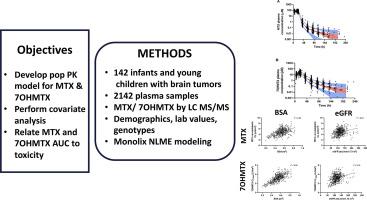
The objectives of this study were to develop a population pharmacokinetic model of methotrexate (MTX) and its primary metabolite 7-hydroxymethotrexate (7OHMTX) in children with brain tumors, to identify the sources of pharmacokinetic variability, and to assess whether MTX and 7OHMTX systemic exposures were related to toxicity.
Methods
Patients received 2.5 or 5 g/m2 MTX as a 24-hour infusion and serial samples were analyzed for MTX and 7OHMTX by an LC-MS/MS method. Pharmacokinetic parameters were estimated using nonlinear mixed-effects modeling. Demographics, laboratory values, and genetic polymorphisms were considered as potential covariates to explain the pharmacokinetic variability. Association between MTX and 7OHMTX systemic exposures and MTX-related toxicities were explored using random intercept logistic regression models.
Results
The population pharmacokinetics of MTX and 7OHMTX were adequately characterized using two-compartment models in 142 patients (median 1.91 y; age range 0.09 to 4.94 y) in 513 courses. The MTX and 7OHMTX population clearance values were 4.6 and 3.0 l/h/m2, respectively. Baseline body surface area and estimated glomerular filtration rate were significant covariates on both MTX and 7OHMTX plasma disposition. Pharmacogenetic genotypes were associated with MTX pharmacokinetic parameters but had only modest influence. No significant association was observed between MTX or 7OHMTX exposure and MTX-related toxicity.
Conclusions
MTX and 7OHMTX plasma disposition were characterized for the first time in young children with brain tumors. No exposure-toxicity relationship was identified in this study, presumably due to aggressive clinical management which led to a low MTX-related toxicity rate.
中文翻译:

甲氨蝶呤和7-羟基甲氨蝶呤在脑肿瘤婴幼儿中的群体药代动力学和延迟排泄
目的
本研究的目的是开发脑肿瘤儿童中甲氨蝶呤 (MTX) 及其主要代谢物 7-羟基甲氨蝶呤 (7OHMTX) 的群体药代动力学模型,以确定药代动力学变异的来源,并评估 MTX 和 7OHMTX 是否存在全身暴露与毒性有关。
方法
患者接受2.5或5 g/m 2 MTX 24小时输注,并通过LC-MS/MS方法分析系列样品中的MTX和7OHMTX。使用非线性混合效应模型估计药代动力学参数。人口统计学、实验室值和遗传多态性被认为是解释药代动力学变异性的潜在协变量。使用随机截距逻辑回归模型探讨了 MTX 和 7OHMTX 全身暴露与 MTX 相关毒性之间的关联。
结果
使用二室模型在 513 个疗程的 142 名患者(中位年龄 1.91 岁;年龄范围 0.09 至 4.94 岁)中充分表征了 MTX 和 7OHMTX 的群体药代动力学。 MTX和7OHMTX群体清除率值分别为4.6和3.0l/h/m 2 。基线体表面积和估计肾小球滤过率是 MTX 和 7OHMTX 血浆分布的显着协变量。药物遗传学基因型与 MTX 药代动力学参数相关,但影响不大。 MTX 或 7OHMTX 暴露与 MTX 相关毒性之间没有观察到显着关联。
结论
首次在患有脑肿瘤的幼儿中对 MTX 和 7OHMTX 血浆分布进行了表征。本研究未发现暴露-毒性关系,可能是由于积极的临床管理导致 MTX 相关毒性率较低。































 京公网安备 11010802027423号
京公网安备 11010802027423号