当前位置:
X-MOL 学术
›
Electrochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Di-chlorinated benzothiadiazole unit: a new strong electron-withdrawing ability acceptor toward fast-switching green low band gap D-A electrochromic polymers
Electrochimica Acta ( IF 5.5 ) Pub Date : 2023-12-10 , DOI: 10.1016/j.electacta.2023.143654
Daize Mo , Tong Tong , Kuirong Deng , Qi Feng
Electrochimica Acta ( IF 5.5 ) Pub Date : 2023-12-10 , DOI: 10.1016/j.electacta.2023.143654
Daize Mo , Tong Tong , Kuirong Deng , Qi Feng
|
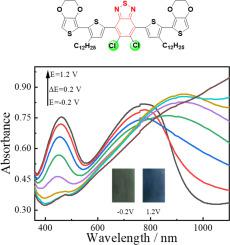
Di-halogenated benzothiadiazole (BT) is one of the potential acceptor units in constructing low band gap d-A polymers, but it has not attracted widespread attention, especially for the di-chlorinated BT unit. Herein, in this work, two kinds of d-π-A-π-D electrochromic conjugated precursors with di-halogenated BT (5,6-dichlorobenzo[][1,2,5]thiadiazole and 5‑chloro-6-fluorobenzo[][1,2,5]thiadiazole) as the acceptor unit, 3-dodecyl thiophene as the π bridge, and 3,4-ethylenedioxythiophene (EDOT) as the donor unit, were designed and synthesized, and their corresponding d-A polymers were successfully obtained by electrochemical deposition method. The photophysical study proves that introducing more chlorine atoms among the precursors will lead to stronger steric hindrance, lower HOMO energy level, blue-shifted absorption spectra, lower quantum yield, but larger Stokes shift. Both of them show very low oxidation potential, which is beneficial for them to obtain high-quality d-A polymers. The introduction of di-chlorinated BT unit obviously improved the polymers’ electroactivity and redox stability, and the remained activity of P(ClCl) was above 74.79 after 1000 cycles. The optical band gap of P(ClCl) is further reduced to 1.28 eV, and the reversible transition from the neutral dark green to the oxidized dark blue can be realized in the redox process. As a result, various favorable electrochromic parameters for P(ClCl) were achieved, such as the optical contrast in the near-infrared region is 27.23 %, the response time is as low as 0.4 s, and the coloration efficiency is as high as 151.3 cm C, as well as the good optical stability and satisfied memory effect. Overall, the subtle change of halogen atoms in BT units is a very efficient method to modulate the photophysical properties of d-π-A-π-D precursors, also providing good guidelines in designing highly efficient d-A electrochromic polymers.
中文翻译:

二氯化苯并噻二唑单元:一种新型强吸电子受体,用于快速切换绿色低带隙DA电致变色聚合物
二卤代苯并噻二唑(BT)是构建低带隙dA聚合物的潜在受体单元之一,但尚未引起广泛关注,特别是二氯化BT单元。本工作中,两种d-π-A-π-D电致变色共轭前驱体与二卤代BT(5,6-二氯苯并[][1,2,5]噻二唑和5-氯-6-氟苯并设计并合成了[][1,2,5]噻二唑)为受体单元,3-十二烷基噻吩为π桥,3,4-乙烯二氧噻吩(EDOT)为供体单元,并合成了相应的dA聚合物采用电化学沉积法成功获得。光物理研究证明,在前驱体中引入更多的氯原子会导致更强的空间位阻,更低的HOMO能级,吸收光谱蓝移,更低的量子产率,但更大的斯托克斯位移。它们都表现出非常低的氧化电位,这有利于它们获得高质量的dA聚合物。二氯化BT单元的引入明显提高了聚合物的电活性和氧化还原稳定性,1000次循环后P(ClCl)的剩余活性在74.79以上。 P(ClCl)的光学带隙进一步减小至1.28 eV,并且在氧化还原过程中可以实现从中性深绿色到氧化深蓝色的可逆跃迁。结果表明,P(ClCl)具有多种良好的电致变色参数,例如近红外区光学对比度为27.23%,响应时间低至0.4 s,着色效率高达151.3 cm C,以及良好的光学稳定性和满意的记忆效果。总体而言,BT单元中卤素原子的微妙变化是调节d-π-A-π-D前驱体光物理性质的一种非常有效的方法,也为设计高效dA电致变色聚合物提供了良好的指导。
更新日期:2023-12-10
中文翻译:

二氯化苯并噻二唑单元:一种新型强吸电子受体,用于快速切换绿色低带隙DA电致变色聚合物
二卤代苯并噻二唑(BT)是构建低带隙dA聚合物的潜在受体单元之一,但尚未引起广泛关注,特别是二氯化BT单元。本工作中,两种d-π-A-π-D电致变色共轭前驱体与二卤代BT(5,6-二氯苯并[][1,2,5]噻二唑和5-氯-6-氟苯并设计并合成了[][1,2,5]噻二唑)为受体单元,3-十二烷基噻吩为π桥,3,4-乙烯二氧噻吩(EDOT)为供体单元,并合成了相应的dA聚合物采用电化学沉积法成功获得。光物理研究证明,在前驱体中引入更多的氯原子会导致更强的空间位阻,更低的HOMO能级,吸收光谱蓝移,更低的量子产率,但更大的斯托克斯位移。它们都表现出非常低的氧化电位,这有利于它们获得高质量的dA聚合物。二氯化BT单元的引入明显提高了聚合物的电活性和氧化还原稳定性,1000次循环后P(ClCl)的剩余活性在74.79以上。 P(ClCl)的光学带隙进一步减小至1.28 eV,并且在氧化还原过程中可以实现从中性深绿色到氧化深蓝色的可逆跃迁。结果表明,P(ClCl)具有多种良好的电致变色参数,例如近红外区光学对比度为27.23%,响应时间低至0.4 s,着色效率高达151.3 cm C,以及良好的光学稳定性和满意的记忆效果。总体而言,BT单元中卤素原子的微妙变化是调节d-π-A-π-D前驱体光物理性质的一种非常有效的方法,也为设计高效dA电致变色聚合物提供了良好的指导。





































 京公网安备 11010802027423号
京公网安备 11010802027423号