Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2023-12-10 , DOI: 10.1016/j.bbamem.2023.184262
Md Zobayer Hossain 1 , Wylie Stroberg 1
|
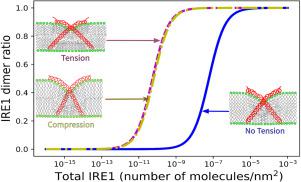
The endoplasmic reticulum acts as a protein quality control center where a range of chaperones and foldases facilitates protein folding. IRE1 is a sensory transmembrane protein that transduces signals of proteotoxic stress by forming clusters and activating a cellular program called the unfolded protein response (UPR). Recently, membrane thickness variation due to membrane compositional changes have been shown to drive IRE1 cluster formation, activating the UPR even in the absence of proteotoxic stress. Here, we demonstrate a direct relationship between bilayer tension and UPR activation based on IRE1 dimer stability. The stability of the IRE1 dimer in a (50%DOPC-50%POPC) membrane at different applied bilayer tensions was analyzed via molecular dynamics simulations. The potential of mean force for IRE1 dimerization predicts a higher concentration of IRE1 dimers for both tensed and compressed ER membranes. This study shows that IRE1 may be a mechanosensitive membrane protein and establishes a direct biophysical relationship between bilayer tension and UPR activation.
中文翻译:

UPR 传感器 IRE1 的双层张力诱导聚集
内质网充当蛋白质质量控制中心,一系列伴侣和折叠酶促进蛋白质折叠。 IRE1 是一种感觉跨膜蛋白,通过形成簇并激活称为未折叠蛋白反应 (UPR) 的细胞程序来转导蛋白毒性应激信号。最近,由于膜组成变化引起的膜厚度变化已被证明会驱动 IRE1 簇的形成,即使在没有蛋白毒性应激的情况下也能激活 UPR。在这里,我们证明了基于 IRE1 二聚体稳定性的双层张力和 UPR 激活之间的直接关系。通过分子动力学模拟分析 (50%DOPC-50%POPC) 膜中 IRE1 二聚体在不同施加双层张力下的稳定性。IRE1 二聚化的平均力的潜力预示着张紧和压缩的 ER 膜的 IRE1 二聚体浓度较高。这项研究表明,IRE1 可能是一种机械敏感的膜蛋白,并在双层张力和 UPR 激活之间建立直接的生物物理关系。


































 京公网安备 11010802027423号
京公网安备 11010802027423号